Abstract
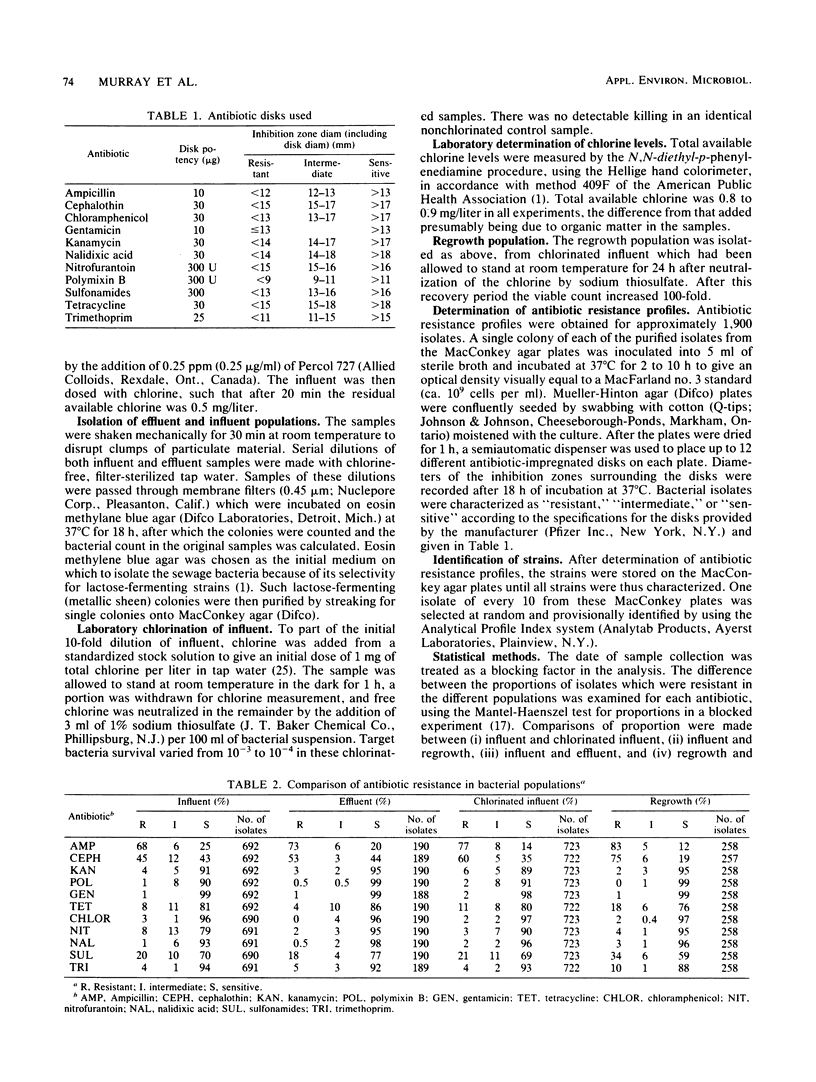
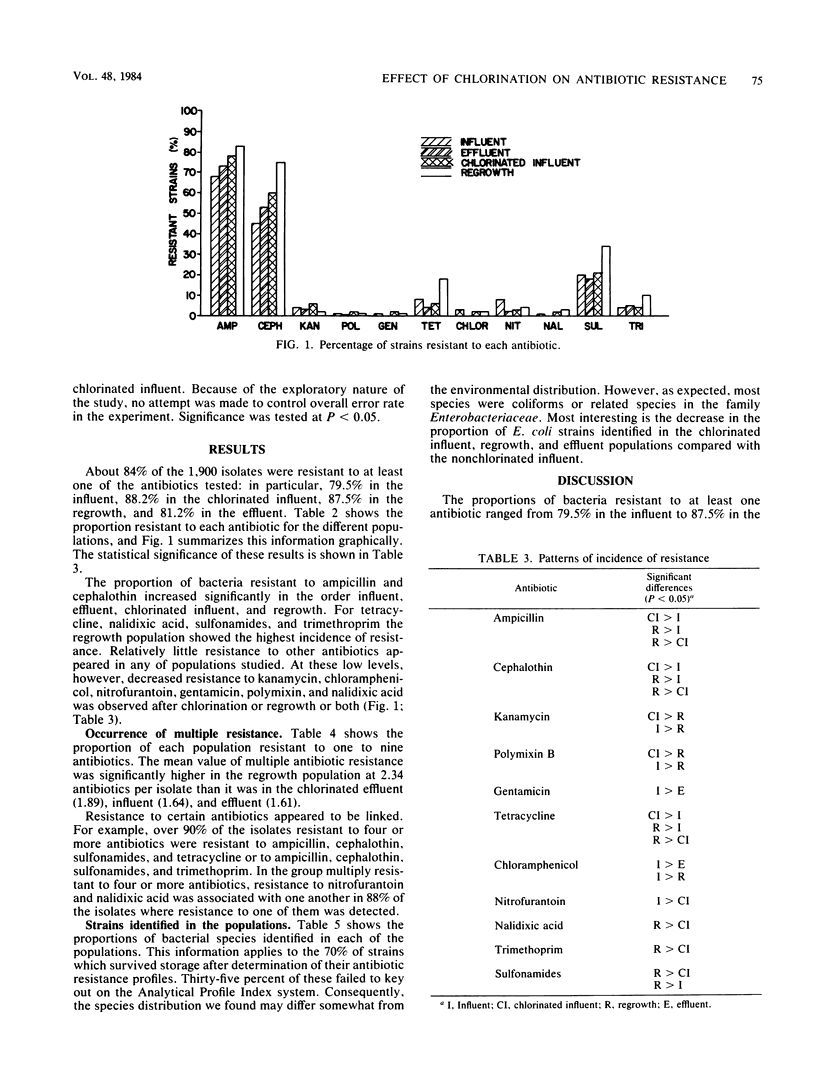
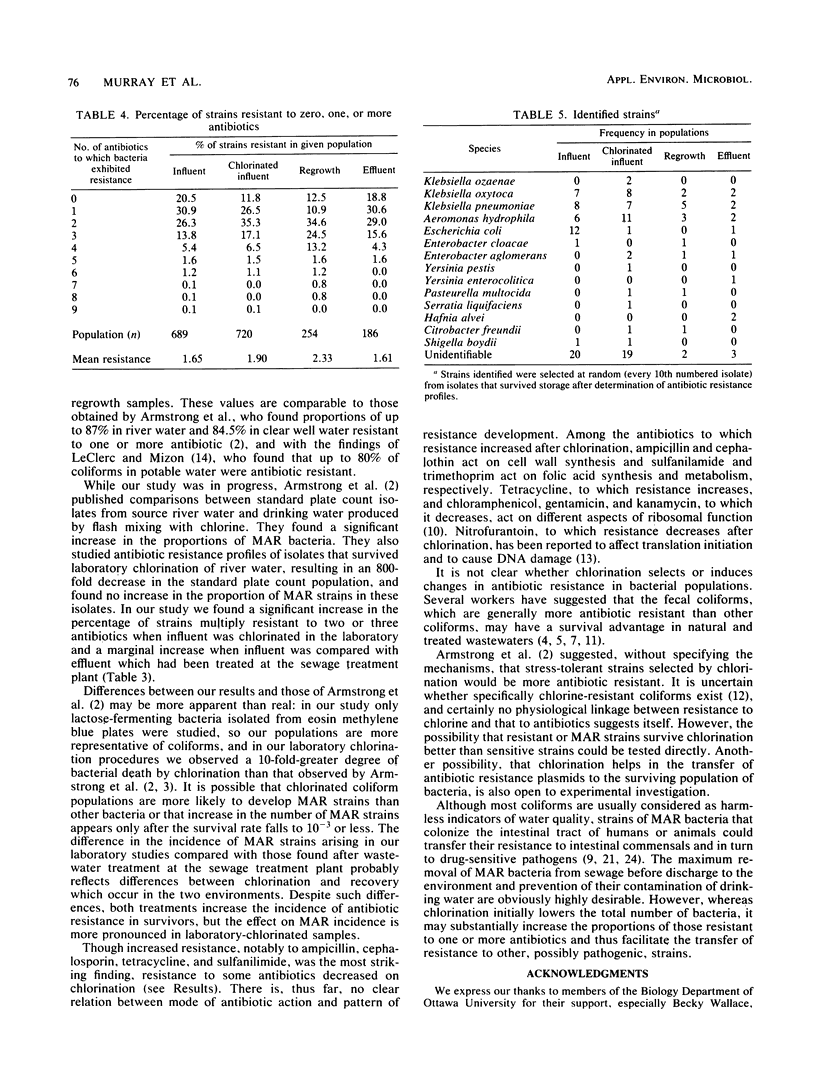
A total of 1,900 lactose-fermenting bacteria were isolated from raw sewage influent and chlorinated sewage effluent from a sewage treatment plant, as well as from chlorinated and neutralized dilute sewage, before and after a 24-h regrowth period in the laboratory. Of these isolates, 84% were resistant to one or more antibiotics. Chlorination of influent resulted in an increase in the proportion of bacteria resistant to ampicillin and cephalothin, the increase being most marked after regrowth occurred following chlorination. Of the other nine antibiotics tested, chlorination resulted in an increased proportion of bacteria resistant to some, but a decrease in the proportion resistant to the remainder. Multiple resistance was found for up to nine antibiotics, especially in regrowth populations. Identification of about 5% of the isolates showed that the highest proportion of Escherichia coli fell in untreated sewage. Some rare and potentially pathogenic species were isolated from chlorinated and regrowth samples, including Yersinia enterocolitica, Yersinia pestis, Pasteurella multocida, and Hafnia alvei. Our results indicate that chlorination, while initially lowering the total number of bacteria in sewage, may substantially increase the proportions of antibiotic-resistant, potentially pathogenic organisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. L., Calomiris J. J., Seidler R. J. Selection of antibiotic-resistant standard plate count bacteria during water treatment. Appl Environ Microbiol. 1982 Aug;44(2):308–316. doi: 10.1128/aem.44.2.308-316.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. L., Shigeno D. S., Calomiris J. J., Seidler R. J. Antibiotic-resistant bacteria in drinking water. Appl Environ Microbiol. 1981 Aug;42(2):277–283. doi: 10.1128/aem.42.2.277-283.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. B., Macrae W. R., Elliott G. E. Incidence of R factors in coliform, fecal coliform, and Salmonella populations of the Red River in Canada. Appl Environ Microbiol. 1980 Sep;40(3):486–491. doi: 10.1128/aem.40.3.486-491.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. B., Macrae W. R., Elliott G. E. R factors in coliform-fecal coliform sewage flora of the prairies and Northwest Territories of Canada. Appl Environ Microbiol. 1981 Aug;42(2):204–210. doi: 10.1128/aem.42.2.204-210.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. B. Antibiotic resistance patterns of fecal coliforms isolated from domestic sewage before and after treatment in an aerobic lagoon. Can J Microbiol. 1978 Jul;24(7):886–888. doi: 10.1139/m78-147. [DOI] [PubMed] [Google Scholar]
- Datta N. Drug resistance and R factors in the bowel bacteria of London patients before and after admission to hospital. Br Med J. 1969 May 17;2(5654):407–411. doi: 10.1136/bmj.2.5654.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. E., Jr, Eidson M., Guerry P., Falkow S., Drusin L. M., Roberts R. B. Interbacterial transfer of R factor in the human intestine: in-vivo acquisition of R-factor-mediated kanamycin resistance by a multiresistant strain of Shigella sonnei. J Infect Dis. 1972 Jul;126(1):27–33. doi: 10.1093/infdis/126.1.27. [DOI] [PubMed] [Google Scholar]
- Herrlich P., Schweiger M. Nitrofurans, a group of synthetic antibiotics, with a new mode of action: discrimination of specific messenger RNA classes. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3386–3390. doi: 10.1073/pnas.73.10.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc H., Mizon F. Eaux d'alimentation et bactéries résistantes aux antibiotiques. Incidences sur les normes. Rev Epidemiol Sante Publique. 1978;26(2):137–146. [PubMed] [Google Scholar]
- Linton K. B., Lee P. A., Richmond M. H., Gillespie W. A., Rowland A. J., Baker V. N. Antibiotic resistance and transmissible R-factors in the intestinal coliform flora of healthy adults and children in an urban and a rural community. J Hyg (Lond) 1972 Mar;70(1):99–104. doi: 10.1017/s0022172400022130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton K. B., Richmond M. H., Bevan R., Gillespie W. A. Antibiotic resistance and R factors in coliform bacilli isolated from hospital and domestic sewage. J Med Microbiol. 1974 Feb;7(1):91–103. doi: 10.1099/00222615-7-1-91. [DOI] [PubMed] [Google Scholar]
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- Meckes M. C. Effect of UV light disinfection on antibiotic-resistant coliforms in wastewater effluents. Appl Environ Microbiol. 1982 Feb;43(2):371–377. doi: 10.1128/aem.43.2.371-377.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhouse E. C. Transferable drug resistance in enterobacteria isolated from urban infants. Br Med J. 1969 May 17;2(5654):405–407. doi: 10.1136/bmj.2.5654.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. Incidence of R + Escherichia coli in coastal bathing waters of Britain. Nature. 1971 Nov 19;234(5325):155–156. doi: 10.1038/234155a0. [DOI] [PubMed] [Google Scholar]
- Smith H. W. Transfer of antibiotic resistance from animal and human strains of Escherichia coli to resident E. coli in the alimentary tract of man. Lancet. 1969 Jun 14;1(7607):1174–1176. doi: 10.1016/s0140-6736(69)92164-3. [DOI] [PubMed] [Google Scholar]
- Sturtevant A. B., Cassell G. H., Feary T. W. Incidence of infectious drug resistance among fecal coliforms isolated from raw sewage. Appl Microbiol. 1971 Mar;21(3):487–491. doi: 10.1128/am.21.3.487-491.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Graevenitz A. The role of opportunistic bacteria in human disease. Annu Rev Microbiol. 1977;31:447–471. doi: 10.1146/annurev.mi.31.100177.002311. [DOI] [PubMed] [Google Scholar]


