Abstract
Herpes simplex virus type 1 glycoprotein B (gB) is essential for virus entry, an event involving fusion of the virus envelope with the cell surface membrane, and virus-induced cell-cell fusion, resulting in polykaryocyte, or syncytium, formation. The experiments described in this report employed a random mutagenesis strategy to develop a more complete genetic map of mutations resulting in the syn mutant phenotype. The results indicate that syn mutations occur within two essential and highly conserved hydrophilic, alpha-helical regions of the gB cytoplasmic domain. Region I is immediately proximal to the transmembrane domain and includes residues R796 to E816/817. Region II is localized centrally in the cytoplasmic domain and includes residues A855 and R858. Positively charged residues were particularly affected in both regions, suggesting that charge interactions may be required to suppress the syn mutant phenotype. No syn mutations were identified within the transmembrane domain. A virus containing a rate of entry (roe) mutation at residue A851, either within or immediately proximal to syn region II, was isolated. Since roe mutations have also been discovered in the external domain of gB, it appears likely that the external and cytoplasmic domains cooperate in virus penetration. Moreover, the observation that both roe and syn mutations occur in the cytoplasmic domain further suggests that gB functions in an analogous manner in both membrane fusion events. It might be predicted from these observations that membrane fusion involves transduction of a fusion signal along the gB molecule through the transmembrane domain. Communication between the external and cytoplasmic domain may thus be required for gB-mediated membrane fusion events.
Full text
PDF



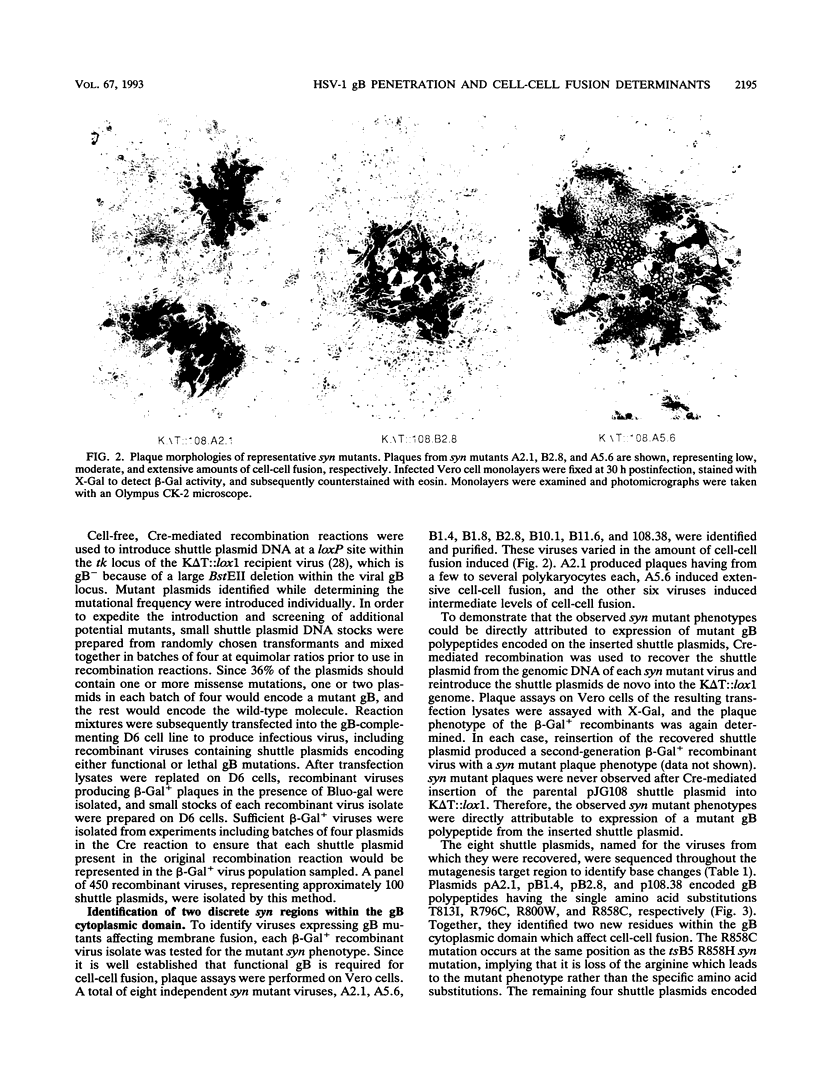
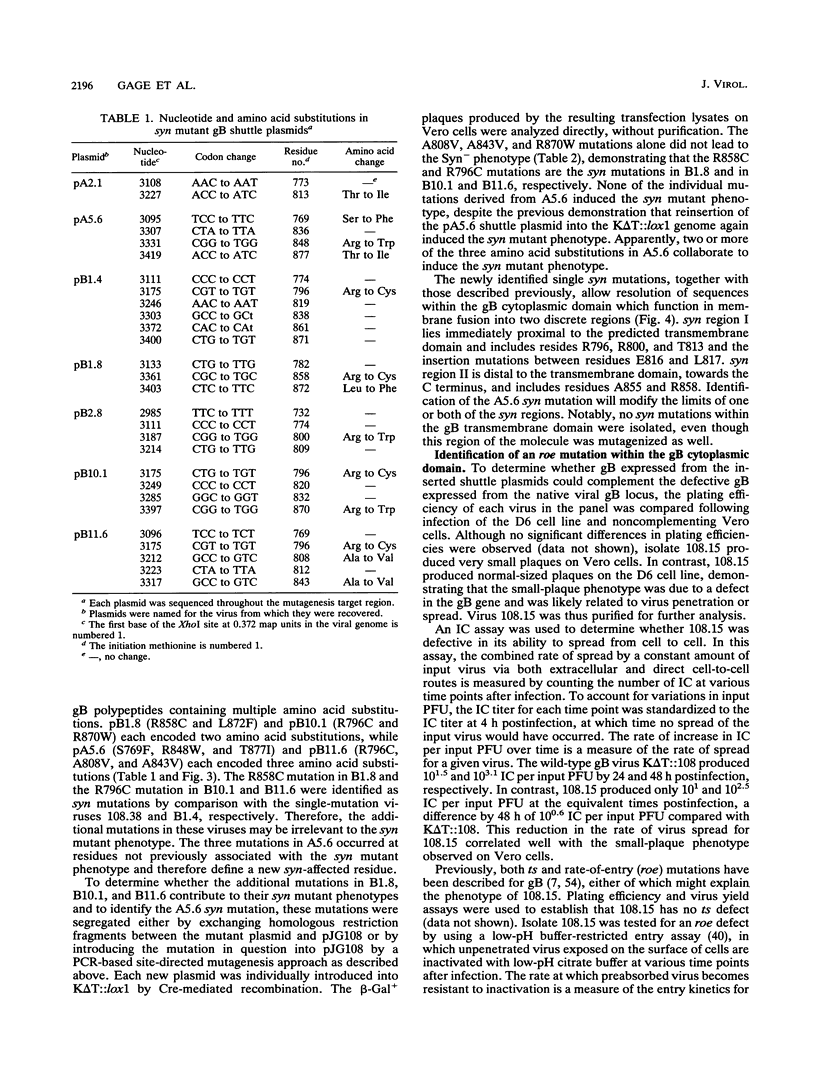
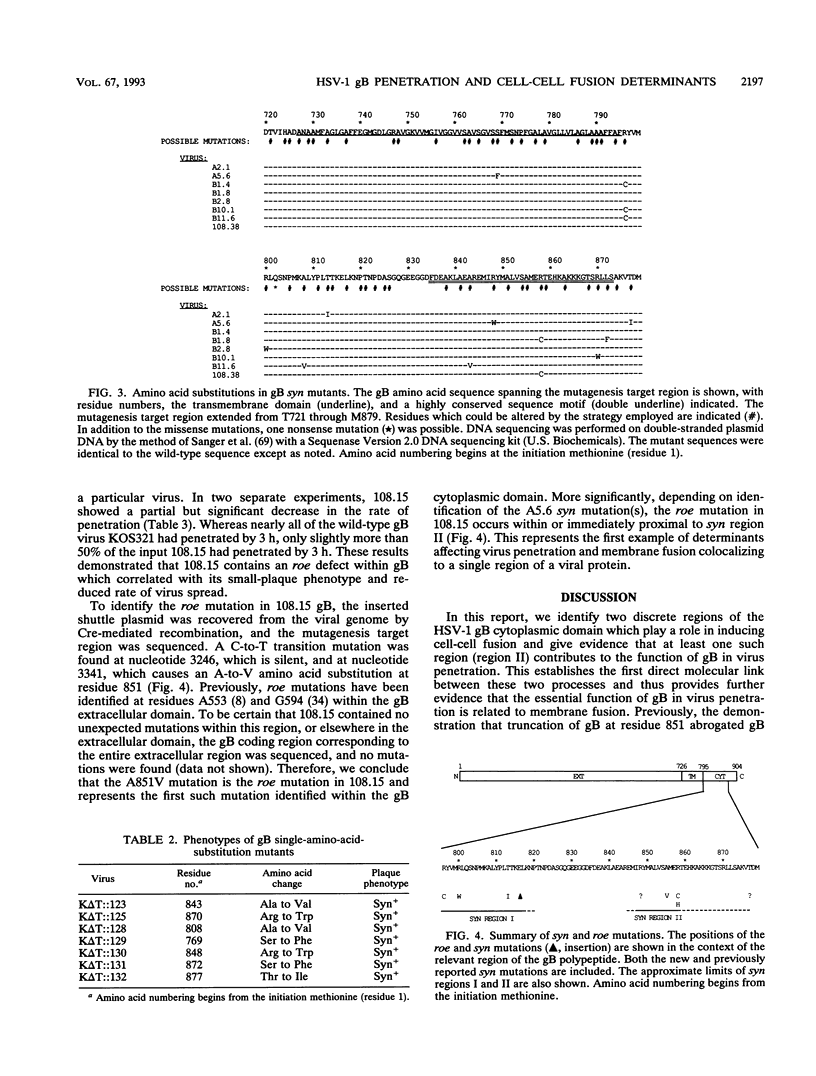
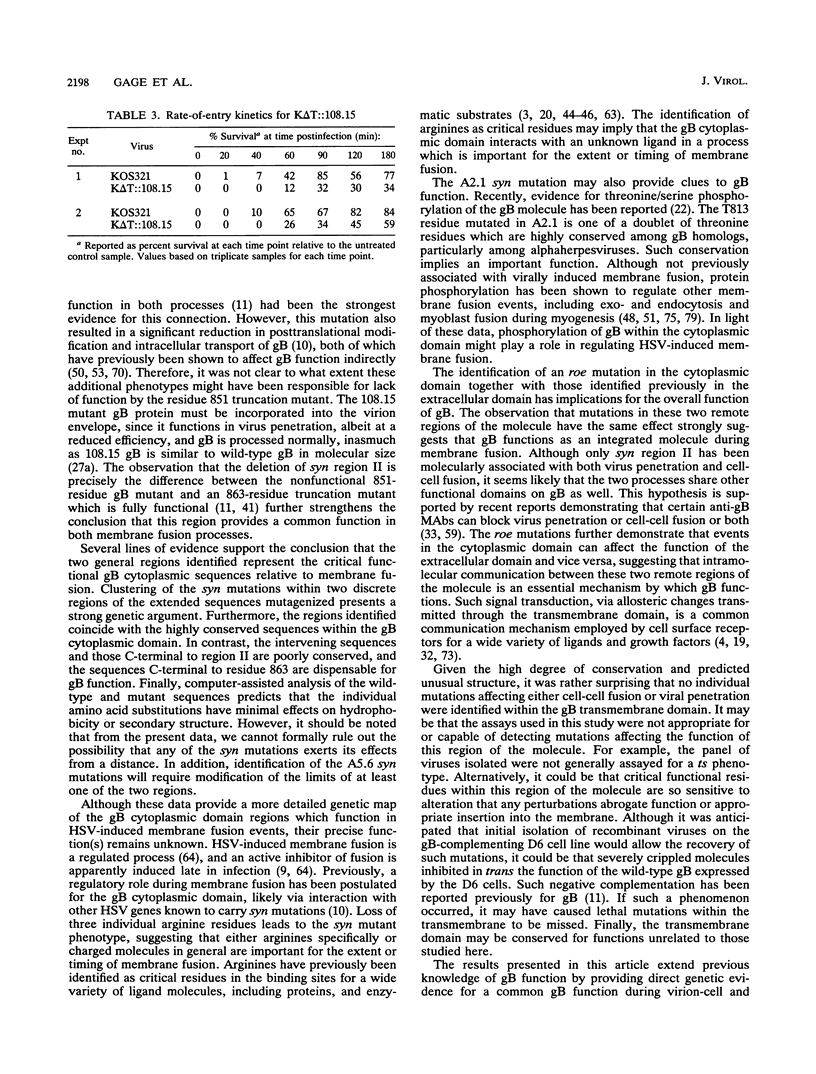



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abremski K., Hoess R. Bacteriophage P1 site-specific recombination. Purification and properties of the Cre recombinase protein. J Biol Chem. 1984 Feb 10;259(3):1509–1514. [PubMed] [Google Scholar]
- Addison C., Rixon F. J., Palfreyman J. W., O'Hara M., Preston V. G. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology. 1984 Oct 30;138(2):246–259. doi: 10.1016/0042-6822(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Borders C. L., Jr, Riordan J. F. An essential arginyl residue at the nucleotide binding site of creatine kinase. Biochemistry. 1975 Oct 21;14(21):4699–4704. doi: 10.1021/bi00692a021. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Debroy C., Fox B. A., Pederson N. E., Person S. The nucleotide sequence of the gB glycoprotein gene of HSV-2 and comparison with the corresponding gene of HSV-1. Virology. 1986 Dec;155(2):322–333. doi: 10.1016/0042-6822(86)90196-0. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology. 1984 Aug;137(1):185–190. doi: 10.1016/0042-6822(84)90022-9. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Person S., Read G. S. The active inhibition of herpes simplex virus type 1-induced cell fusion. Virology. 1982 Mar;117(2):504–509. doi: 10.1016/0042-6822(82)90490-1. [DOI] [PubMed] [Google Scholar]
- Cai W. H., Gu B., Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988 Aug;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. Z., Person S., DebRoy C., Gu B. H. Functional regions and structural features of the gB glycoprotein of herpes simplex virus type 1. An analysis of linker insertion mutants. J Mol Biol. 1988 Jun 5;201(3):575–588. doi: 10.1016/0022-2836(88)90639-0. [DOI] [PubMed] [Google Scholar]
- Cai W. Z., Person S., Warner S. C., Zhou J. H., DeLuca N. A. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J Virol. 1987 Mar;61(3):714–721. doi: 10.1128/jvi.61.3.714-721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Arsenakis M., Farabegoli F., Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988 Jan;62(1):159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N., Bzik D. J., Bond V. C., Person S., Snipes W. Nucleotide sequences of herpes simplex virus type 1 (HSV-1) affecting virus entry, cell fusion, and production of glycoprotein gb (VP7). Virology. 1982 Oct 30;122(2):411–423. doi: 10.1016/0042-6822(82)90240-9. [DOI] [PubMed] [Google Scholar]
- DeLuca N., Bzik D., Person S., Snipes W. Early events in herpes simplex virus type 1 infection: photosensitivity of fluorescein isothiocyanate-treated virions. Proc Natl Acad Sci U S A. 1981 Feb;78(2):912–916. doi: 10.1073/pnas.78.2.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P. J., Schaffer P. A., Minson A. C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988 Jun;69(Pt 6):1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Savage N. Interleukin-1 and its receptor. Crit Rev Immunol. 1989;9(1):1–20. [PubMed] [Google Scholar]
- Duerksen-Hughes P. J., Xu X. X., Wilkinson K. D. Structure and function of ubiquitin: evidence for differential interactions of arginine-74 with the activating enzyme and the proteases of ATP-dependent proteolysis. Biochemistry. 1987 Nov 3;26(22):6980–6987. doi: 10.1021/bi00396a019. [DOI] [PubMed] [Google Scholar]
- Eberle R., Black D., Hilliard J. K. Relatedness of glycoproteins expressed on the surface of simian herpes-virus virions and infected cells to specific HSV glycoproteins. Arch Virol. 1989;109(3-4):233–252. doi: 10.1007/BF01311084. [DOI] [PubMed] [Google Scholar]
- Edson C. M., Hosler B. A., Waters D. J. Varicella-zoster virus gpI and herpes simplex virus gE: phosphorylation and Fc binding. Virology. 1987 Dec;161(2):599–602. doi: 10.1016/0042-6822(87)90157-7. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Forrester A., Farrell H., Wilkinson G., Kaye J., Davis-Poynter N., Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992 Jan;66(1):341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Santos R. E., Spear P. G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989 Aug;63(8):3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. J., Sauer B., Levine M., Glorioso J. C. A cell-free recombination system for site-specific integration of multigenic shuttle plasmids into the herpes simplex virus type 1 genome. J Virol. 1992 Sep;66(9):5509–5515. doi: 10.1128/jvi.66.9.5509-5515.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- HOLMES I. H., WATSON D. H. AN ELECTRON MICROSCOPE STUDY OF THE ATTACHMENT AND PENETRATION OF HERPES VIRUS IN BHK21 CELLS. Virology. 1963 Sep;21:112–123. doi: 10.1016/0042-6822(63)90309-x. [DOI] [PubMed] [Google Scholar]
- HUANG A. S., WAGNER R. R. PENETRATION OF HERPES SIMPLEX VIRUS INTO HUMAN EPIDERMOID CELLS. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- Hayatsu H. Bisulfite modification of nucleic acids and their constituents. Prog Nucleic Acid Res Mol Biol. 1976;16:75–124. doi: 10.1016/s0079-6603(08)60756-4. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. A regulatory hierarchy for cell specialization in yeast. Nature. 1989 Dec 14;342(6251):749–757. doi: 10.1038/342749a0. [DOI] [PubMed] [Google Scholar]
- Highlander S. L., Cai W. H., Person S., Levine M., Glorioso J. C. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J Virol. 1988 Jun;62(6):1881–1888. doi: 10.1128/jvi.62.6.1881-1888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Goins W. F., Person S., Holland T. C., Levine M., Glorioso J. C. Oligomer formation of the gB glycoprotein of herpes simplex virus type 1. J Virol. 1991 Aug;65(8):4275–4283. doi: 10.1128/jvi.65.8.4275-4283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Person S. Ammonium chloride inhibits cell fusion induced by syn mutants of herpes simplex virus type 1. J Virol. 1977 Jul;23(1):213–215. doi: 10.1128/jvi.23.1.213-215.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa F. L., Otal T. M., Glorioso J. C., Levine M. Transcriptional control signals of a herpes simplex virus type 1 late (gamma 2) gene lie within bases -34 to +124 relative to the 5' terminus of the mRNA. Mol Cell Biol. 1986 Nov;6(11):3652–3666. doi: 10.1128/mcb.6.11.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff V., Cai W., Glorioso J. C., Levine M. The carboxy-terminal 41 amino acids of herpes simplex virus type 1 glycoprotein B are not essential for production of infectious virus particles. J Virol. 1988 Nov;62(11):4403–4406. doi: 10.1128/jvi.62.11.4403-4406.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson L., Browne H., Wargent V., Davis-Poynter N., Primorac S., Goldsmith K., Minson A. C., Johnson D. C. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992 Apr;66(4):2240–2250. doi: 10.1128/jvi.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson L., Goldsmith K., Snoddy D., Ghosh H., Graham F. L., Johnson D. C. Identification and characterization of a novel herpes simplex virus glycoprotein, gK, involved in cell fusion. J Virol. 1992 Sep;66(9):5603–5609. doi: 10.1128/jvi.66.9.5603-5609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaikaria N. S., Rosenfeld L., Khan M. Y., Danishefsky I., Newman S. A. Interaction of fibronectin with heparin in model extracellular matrices: role of arginine residues and sulfate groups. Biochemistry. 1991 Feb 12;30(6):1538–1544. doi: 10.1021/bi00220a014. [DOI] [PubMed] [Google Scholar]
- Jorgensen A. M., Borders C. L., Jr, Fish W. W. Arginine residues are critical for the heparin-cofactor activity of antithrombin III. Biochem J. 1985 Oct 1;231(1):59–63. doi: 10.1042/bj2310059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasher J. S., Allen K. E., Kasamo K., Slayman C. W. Characterization of an essential arginine residue in the plasma membrane H+-ATPase of Neurospora crassa. J Biol Chem. 1986 Aug 15;261(23):10808–10813. [PubMed] [Google Scholar]
- Keller P. M., Davison A. J., Lowe R. S., Bennett C. D., Ellis R. W. Identification and structure of the gene encoding gpII, a major glycoprotein of varicella-zoster virus. Virology. 1986 Jul 15;152(1):181–191. doi: 10.1016/0042-6822(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Lee I. H., Chung C. H., Kang M. S., Ha D. B. Ca2+/calmodulin-dependent phosphorylation of the 100-kDa protein in chick embryonic muscle cells in culture. Dev Biol. 1992 Apr;150(2):223–230. doi: 10.1016/0012-1606(92)90237-b. [DOI] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. P., Schaffer P. A. Expression of the syncytial (syn) phenotype in HSV-1, strain KOS: genetic and phenotypic studies of mutants in two syn loci. Virology. 1981 Jul 30;112(2):686–702. doi: 10.1016/0042-6822(81)90314-7. [DOI] [PubMed] [Google Scholar]
- Lognonne J. L., Wahrmann J. P. A cell surface phosphoprotein of 48 kDa specific for myoblast fusion. Cell Differ. 1988 Feb;22(3):245–258. doi: 10.1016/0045-6039(88)90016-4. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Highlander S. L., Holland T. C., Levine M., Glorioso J. C. Antigenic variation (mar mutations) in herpes simplex virus glycoprotein B can induce temperature-dependent alterations in gB processing and virus production. J Virol. 1986 Jul;59(1):142–153. doi: 10.1128/jvi.59.1.142-153.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Misra V., Blewett E. L. Construction of herpes simplex viruses that are pseudodiploid for the glycoprotein B gene: a strategy for studying the function of an essential herpesvirus gene. J Gen Virol. 1991 Feb;72(Pt 2):385–392. doi: 10.1099/0022-1317-72-2-385. [DOI] [PubMed] [Google Scholar]
- Misra V., Nelson R., Smith M. Sequence of a bovine herpesvirus type-1 glycoprotein gene that is homologous to the herpes simplex gene for the glycoprotein gB. Virology. 1988 Oct;166(2):542–549. doi: 10.1016/0042-6822(88)90525-9. [DOI] [PubMed] [Google Scholar]
- Navarro D., Paz P., Pereira L. Domains of herpes simplex virus I glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology. 1992 Jan;186(1):99–112. doi: 10.1016/0042-6822(92)90064-v. [DOI] [PubMed] [Google Scholar]
- Pellett P. E., Biggin M. D., Barrell B., Roizman B. Epstein-Barr virus genome may encode a protein showing significant amino acid and predicted secondary structure homology with glycoprotein B of herpes simplex virus 1. J Virol. 1985 Dec;56(3):807–813. doi: 10.1128/jvi.56.3.807-813.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Kousoulas K. G., Pereira L., Roizman B. Anatomy of the herpes simplex virus 1 strain F glycoprotein B gene: primary sequence and predicted protein structure of the wild type and of monoclonal antibody-resistant mutants. J Virol. 1985 Jan;53(1):243–253. doi: 10.1128/jvi.53.1.243-253.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan L. M., Noltmann E. A. Specific arginine modification at the phosphatase site of muscle carbonic anhydrase. Biochemistry. 1985 Jan 29;24(3):635–640. doi: 10.1021/bi00324a015. [DOI] [PubMed] [Google Scholar]
- Read G. S., Person S., Keller P. M. Genetic studies of cell fusion induced by herpes simplex virus type 1. J Virol. 1980 Jul;35(1):105–113. doi: 10.1128/jvi.35.1.105-113.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggio M. P., Cullinane A. A., Onions D. E. Identification and nucleotide sequence of the glycoprotein gB gene of equine herpesvirus 4. J Virol. 1989 Mar;63(3):1123–1133. doi: 10.1128/jvi.63.3.1123-1133.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Dorney D. J., Wathen M. W., Whealy M. E., Gold C., Watson R. J., Holland L. E., Weed S. D., Levine M., Glorioso J. C. The pseudorabies virus gII gene is closely related to the gB glycoprotein gene of herpes simplex virus. J Virol. 1987 Sep;61(9):2691–2701. doi: 10.1128/jvi.61.9.2691-2701.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. J., Sanderson M., Scott S. D., Binns M. M., Doel T., Milne B. Nucleotide sequence and characterization of the Marek's disease virus homologue of glycoprotein B of herpes simplex virus. J Gen Virol. 1989 Jul;70(Pt 7):1789–1804. doi: 10.1099/0022-1317-70-7-1789. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Local mutagenesis: a method for generating viral mutants with base substitutions in preselected regions of the viral genome. Proc Natl Acad Sci U S A. 1978 May;75(5):2170–2174. doi: 10.1073/pnas.75.5.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storms R. W., Bose H. R., Jr Oncogenes, protooncogenes, and signal transduction: toward a unified theory? Adv Virus Res. 1989;37:1–34. doi: 10.1016/s0065-3527(08)60831-3. [DOI] [PubMed] [Google Scholar]
- Stuve L. L., Brown-Shimer S., Pachl C., Najarian R., Dina D., Burke R. L. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J Virol. 1987 Feb;61(2):326–335. doi: 10.1128/jvi.61.2.326-335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Newsholme P., Cawley K. C., van Patten S. M., Angelos K. L. Motifs of protein phosphorylation and mechanisms of reversible covalent regulation. Physiol Rev. 1991 Jan;71(1):285–304. doi: 10.1152/physrev.1991.71.1.285. [DOI] [PubMed] [Google Scholar]
- Whalley J. M., Robertson G. R., Scott N. A., Hudson G. C., Bell C. W., Woodworth L. M. Identification and nucleotide sequence of a gene in equine herpesvirus 1 analogous to the herpes simplex virus gene encoding the major envelope glycoprotein gB. J Gen Virol. 1989 Feb;70(Pt 2):383–394. doi: 10.1099/0022-1317-70-2-383. [DOI] [PubMed] [Google Scholar]
- Whitbeck J. C., Bello L. J., Lawrence W. C. Comparison of the bovine herpesvirus 1 gI gene and the herpes simplex virus type 1 gB gene. J Virol. 1988 Sep;62(9):3319–3327. doi: 10.1128/jvi.62.9.3319-3327.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Woodman P. G., Mundy D. I., Cohen P., Warren G. Cell-free fusion of endocytic vesicles is regulated by phosphorylation. J Cell Biol. 1992 Jan;116(2):331–338. doi: 10.1083/jcb.116.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]




