Abstract
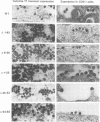
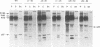
Mason-Pfizer monkey virus (M-PMV) represents the prototype type D retrovirus, characterized by the assembly of intracytoplasmic A-type particles within the infected-cell cytoplasm. These immature particles migrate to the plasma membrane, where they are released by budding. The gag gene of M-PMV encodes a novel protein, p12, just 5' of the major capsid protein (CA) p27 on the polyprotein precursor. The function of p12 is not known, but an equivalent protein is found in mouse mammary tumor virus and is absent from the type C retroviruses. In order to determine whether the p12 protein plays a role in the intracytoplasmic assembly of capsids, a series of in-frame deletion mutations were constructed in the p12 coding domain. The mutant gag genes were expressed by a recombinant vaccinia virus-T7 polymerase-based system in CV-1 cells or in the context of the viral genome in COS-1 cells. In both of these high-level expression systems, mutant Gag precursors were competent to assemble but were not infectious. In contrast, when stable transfectant HeLa cell lines were established, assembly of the mutant precursors into capsids was drastically reduced. Instead, the polyprotein precursors remained predominantly soluble in the cytoplasm. These results show that while p12 is not required for the intracytoplasmic assembly of M-PMV capsids, under the conditions of low-level protein biosynthesis seen in virus-infected cells, it may assist in the stable association of polyprotein precursors for capsid assembly. Moreover, the presence of the p12 coding domain is absolutely required for the infectivity of M-PMV virions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Long L. K., Aaronson S. A. Major structural proteins of type B, type C, and type D oncoviruses share interspecies antigenic determinants. Proc Natl Acad Sci U S A. 1980 Jan;77(1):72–76. doi: 10.1073/pnas.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradac J., Hunter E. Polypeptides of Mason-Pfizer monkey virus. I. Synthesis and processing of the gag-gene products. Virology. 1984 Oct 30;138(2):260–275. doi: 10.1016/0042-6822(84)90350-7. [DOI] [PubMed] [Google Scholar]
- Bryant M. L., Gardner M. B., Marx P. A., Maul D. H., Lerche N. W., Osborn K. G., Lowenstine L. J., Bodgen A., Arthur L. O., Hunter E. Immunodeficiency in rhesus monkeys associated with the original Mason-Pfizer monkey virus. J Natl Cancer Inst. 1986 Oct;77(4):957–965. [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Cochran M. A., Mackett M., Moss B. Eukaryotic transient expression system dependent on transcription factors and regulatory DNA sequences of vaccinia virus. Proc Natl Acad Sci U S A. 1985 Jan;82(1):19–23. doi: 10.1073/pnas.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Hanson R. E., Jr, Stephenson J. R. Primate retroviruses: envelope glycoproteins of endogenous type C and type D viruses possess common interspecies antigenic determinants. J Virol. 1978 May;26(2):316–324. doi: 10.1128/jvi.26.2.316-324.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizi A., Henderson L. E., Copeland T. D., Sowder R. C., Krutzsch H. C., Oroszlan S. Analysis of gag proteins from mouse mammary tumor virus. J Virol. 1989 Jun;63(6):2543–2549. doi: 10.1128/jvi.63.6.2543-2549.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. M., Zelljadt I., Chopra H. C., Mason M. M. Isolation and propagation of a virus from a spontaneous mammary carcinoma of a rhesus monkey. Cancer Res. 1970 Sep;30(9):2388–2393. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik A. K., Wertz G. W. Replication and amplification of defective interfering particle RNAs of vesicular stomatitis virus in cells expressing viral proteins from vectors containing cloned cDNAs. J Virol. 1990 Jun;64(6):2948–2957. doi: 10.1128/jvi.64.6.2948-2957.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hui H. X., Hunter E. Preassembled capsids of type D retroviruses contain a signal sufficient for targeting specifically to the plasma membrane. J Virol. 1990 Aug;64(8):3844–3852. doi: 10.1128/jvi.64.8.3844-3852.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. A single amino acid substitution within the matrix protein of a type D retrovirus converts its morphogenesis to that of a type C retrovirus. Cell. 1990 Oct 5;63(1):77–86. doi: 10.1016/0092-8674(90)90289-q. [DOI] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J Virol. 1987 Apr;61(4):1045–1053. doi: 10.1128/jvi.61.4.1045-1053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Structural role of the matrix protein of type D retroviruses in gag polyprotein stability and capsid assembly. J Virol. 1990 Sep;64(9):4383–4389. doi: 10.1128/jvi.64.9.4383-4389.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfelt M. A., Petteway S. R., Jr, Dreyer G. B., Hunter E. Effect of retroviral proteinase inhibitors on Mason-Pfizer monkey virus maturation and transmembrane glycoprotein cleavage. J Virol. 1992 Jul;66(7):4220–4227. doi: 10.1128/jvi.66.7.4220-4227.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Hino S., Garrett E. W., Aaronson S. A. Immunological cross reactivity of Mason-Pfizer monkey virus with type C RNA viruses endogenous to primates. Nature. 1976 Jun 17;261(5561):609–611. doi: 10.1038/261609a0. [DOI] [PubMed] [Google Scholar]