Abstract
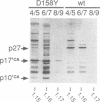
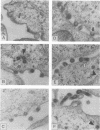
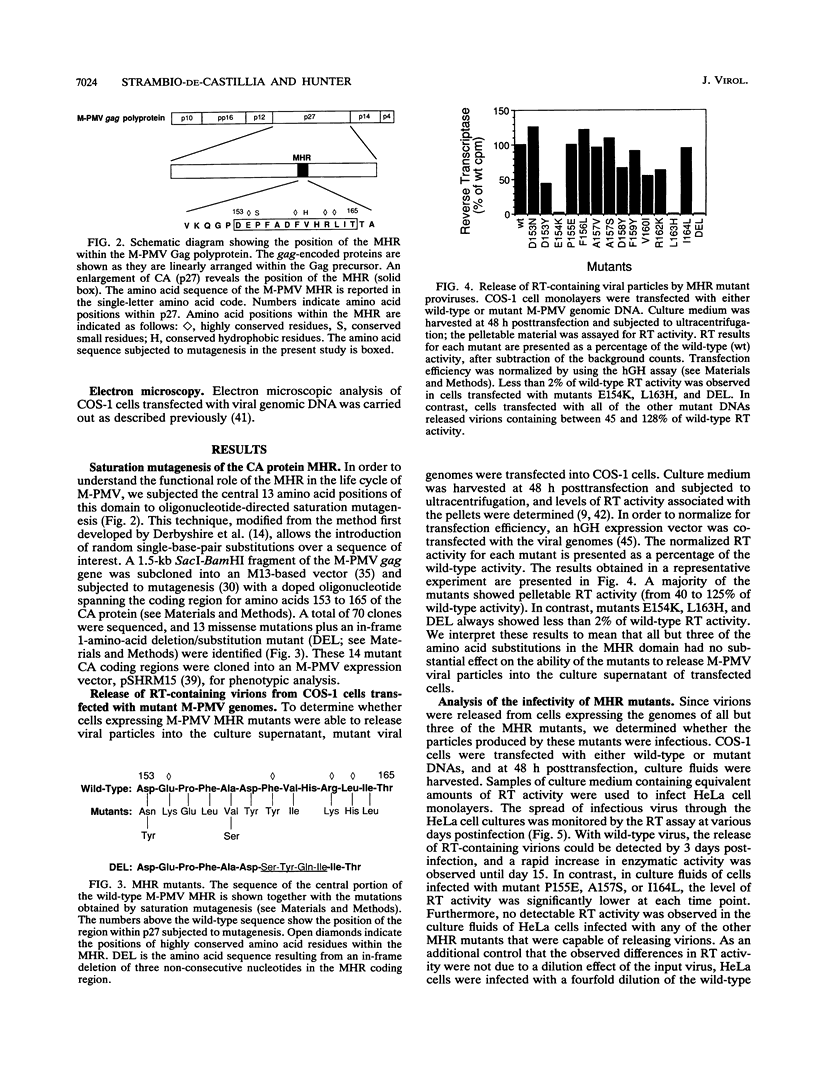
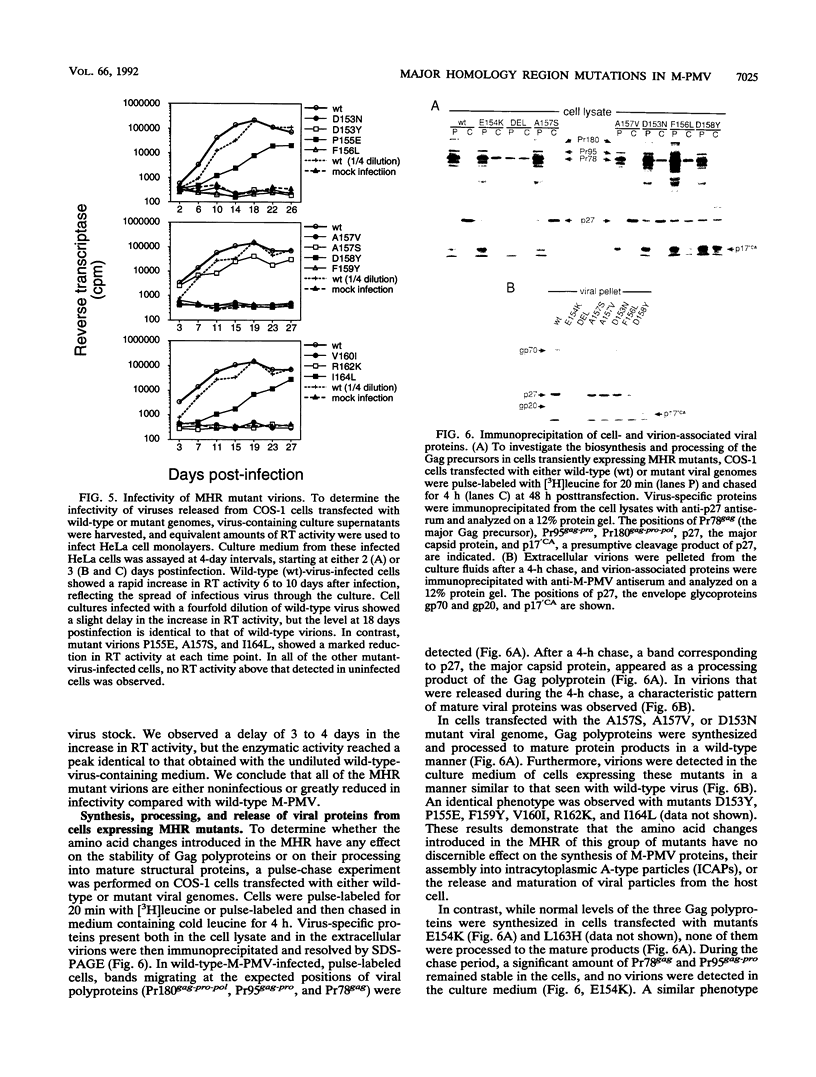
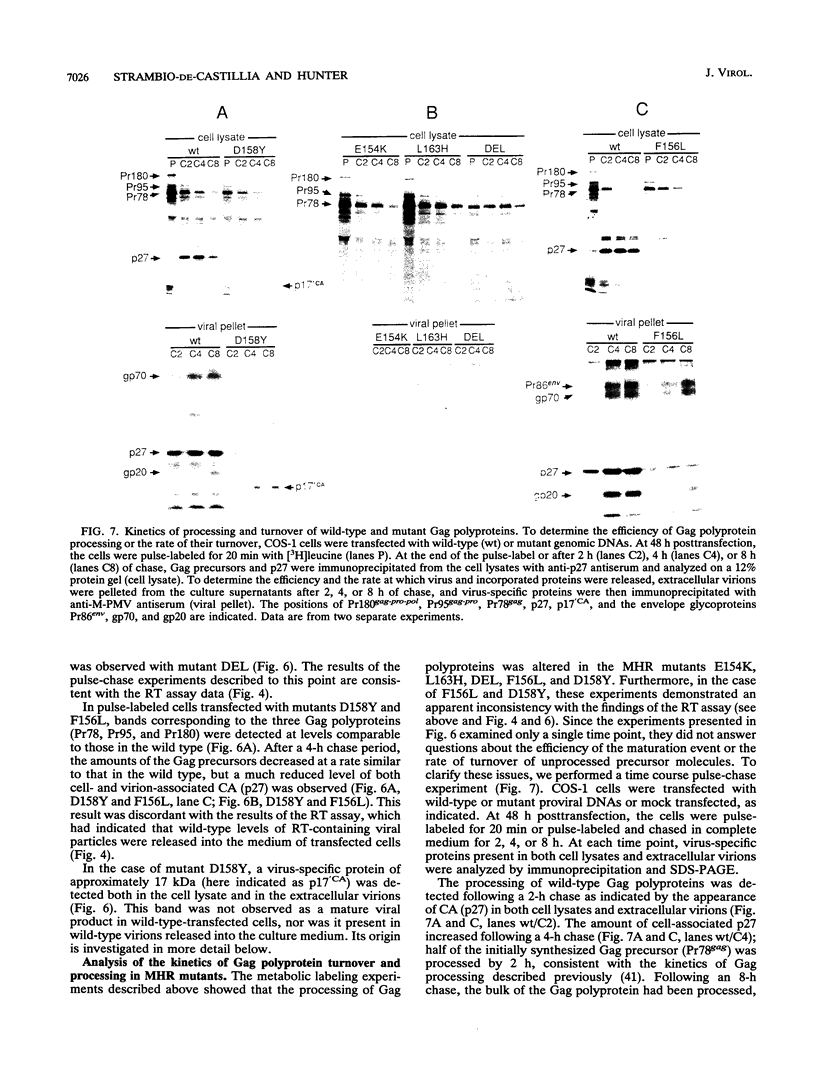
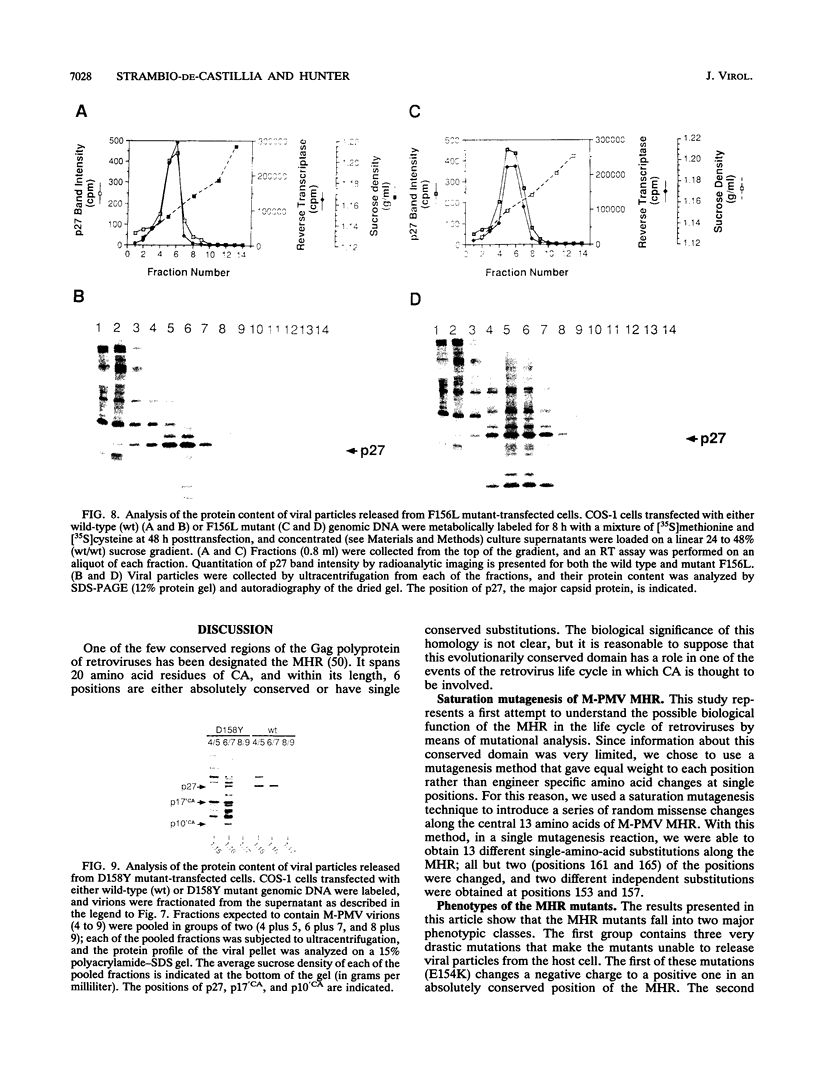
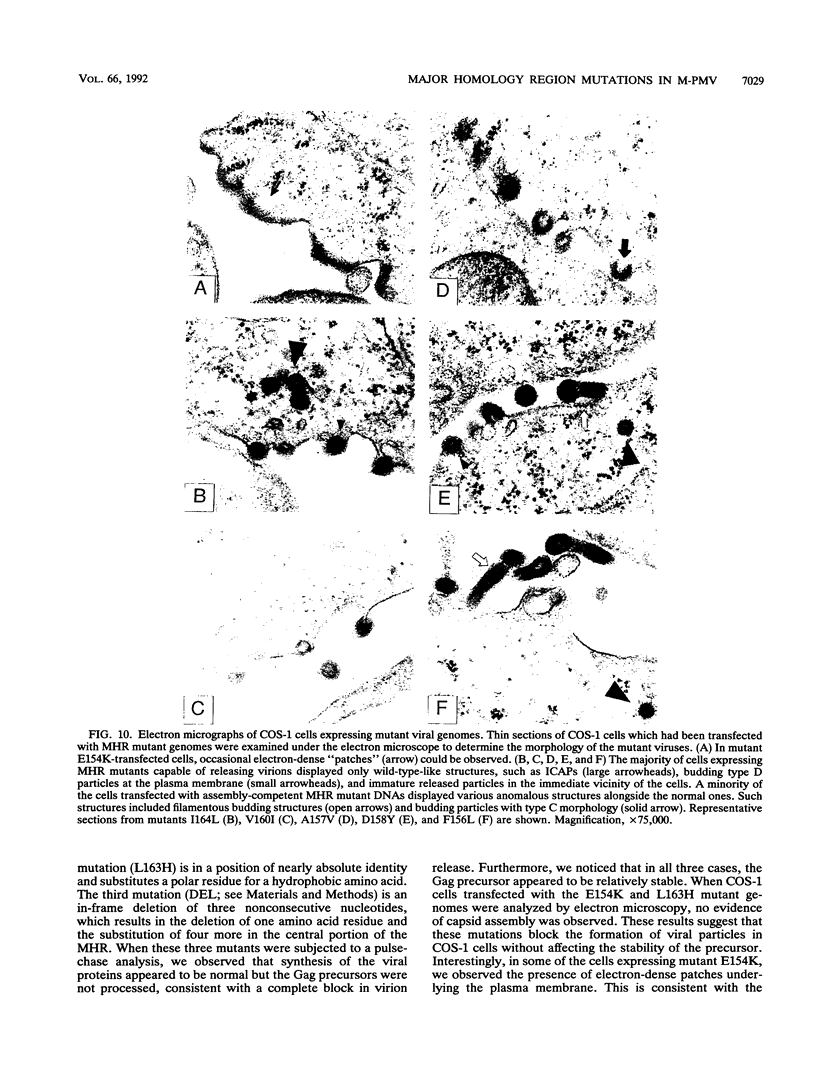
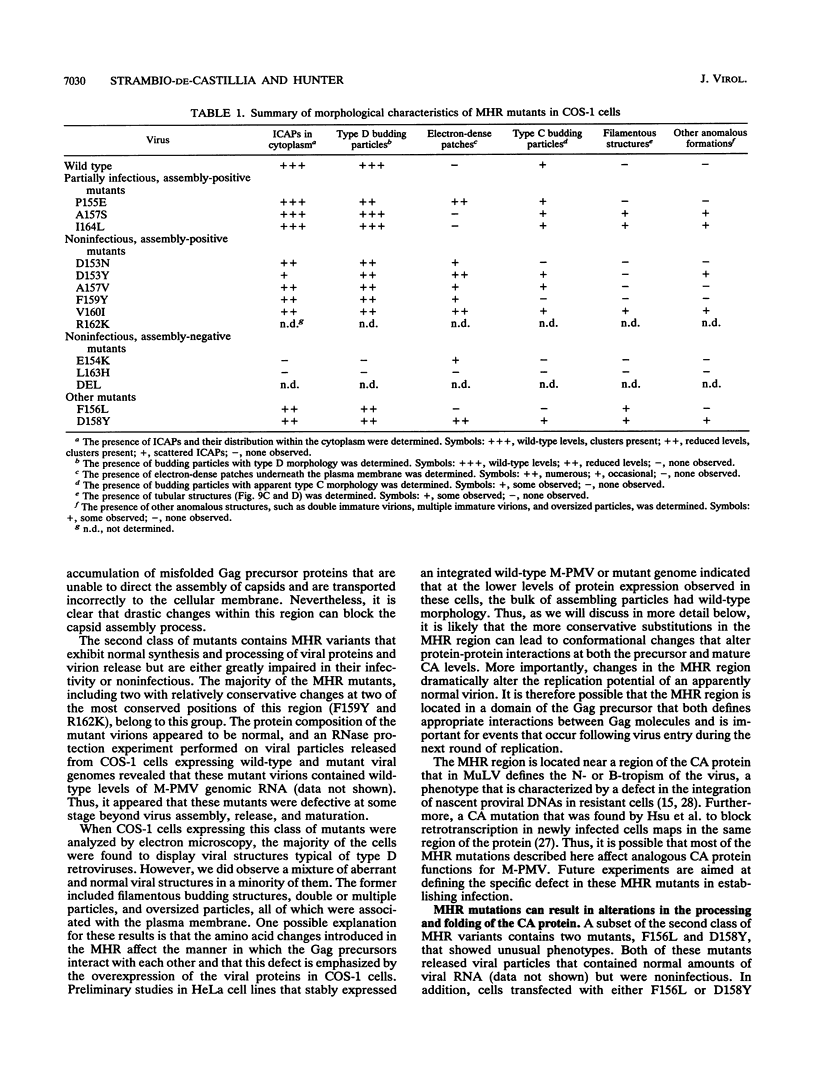
The major capsid (CA) protein of retroviruses possesses a stretch of 20 amino acids, called the major homology region (MHR), which is evolutionarily conserved and invariant in location within the primary sequence of the protein. The function of this region was investigated by examining the effect of random single-amino-acid substitutions within the central 13 positions of the MHR on the life cycle of Mason-Pfizer monkey virus (M-PMV), an immunosuppressive D-type retrovirus. When these mutants were subcloned into an M-PMV proviral vector and expressed in COS cells, one of two major phenotypes was observed. The first group, containing three mutants bearing drastic amino acid substitutions, was unable to assemble capsids in the cytoplasm of the host cell. The second and more common group of mutants was able to assemble and release virions, but these either displayed greatly reduced levels of infectivity or were completely noninfectious. Included within this second group were two mutants with unusual phenotypes; mutant D158Y exhibited a novel cleavage site for the viral protease that resulted in cleavage of the major capsid protein, p27 (CA), within the MHR, whereas mutant F156L appeared to have lost a major site for antibody recognition within the mature CA protein. The results of this mutagenic analysis suggest that changes in the MHR sequence can interfere with the assembly of viral capsids and block an early stage of the infection cycle of M-PMV.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. E., Mellor J., Gull K., Sim R. B., Tuite M. F., Kingsman S. M., Kingsman A. J. The functions and relationships of Ty-VLP proteins in yeast reflect those of mammalian retroviral proteins. Cell. 1987 Apr 10;49(1):111–119. doi: 10.1016/0092-8674(87)90761-6. [DOI] [PubMed] [Google Scholar]
- Argos P. A possible homology between immunodeficiency virus p24 core protein and picornaviral VP2 coat protein: prediction of HIV p24 antigenic sites. EMBO J. 1989 Mar;8(3):779–785. doi: 10.1002/j.1460-2075.1989.tb03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi D. P., Gelderblom H., Bauer H., Mölling K., Hüper G. Polypeptides of avian RNA tumor viruses. V. Analysis of the virus core. Virology. 1972 Mar;47(3):567–578. doi: 10.1016/0042-6822(72)90546-6. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Montelaro R. C., Frank H., Schäfer W. Assembly of type C oncornaviruses: a model. Science. 1978 Jan 13;199(4325):183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- Bowerman B., Brown P. O., Bishop J. M., Varmus H. E. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 1989 Apr;3(4):469–478. doi: 10.1101/gad.3.4.469. [DOI] [PubMed] [Google Scholar]
- Bradac J. A., Hunter E. Polypeptides of Mason-Pfizer monkey virus. III. Translational order of proteins on the gag and env gene specified precursor polypeptides. Virology. 1986 Apr 30;150(2):503–508. doi: 10.1016/0042-6822(86)90314-4. [DOI] [PubMed] [Google Scholar]
- Bradac J., Hunter E. Polypeptides of Mason-Pfizer monkey virus. I. Synthesis and processing of the gag-gene products. Virology. 1984 Oct 30;138(2):260–275. doi: 10.1016/0042-6822(84)90350-7. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Bradac J., Hunter E. A rapid screening procedure for the isolation of nonconditional replication mutants of Mason-Pfizer monkey virus: identification of a mutant defective in pol. Virology. 1985 Feb;141(1):65–76. doi: 10.1016/0042-6822(85)90183-7. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsky J., Soeiro R. Fv-1 host restriction of Friend leukemia virus: analysis of unintegrated proviral DNA. J Virol. 1981 Oct;40(1):45–55. doi: 10.1128/jvi.40.1.45-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Daniel M. D., King N. W., Letvin N. L., Hunt R. D., Sehgal P. K., Desrosiers R. C. A new type D retrovirus isolated from macaques with an immunodeficiency syndrome. Science. 1984 Feb 10;223(4636):602–605. doi: 10.1126/science.6695172. [DOI] [PubMed] [Google Scholar]
- Derbyshire K. M., Salvo J. J., Grindley N. D. A simple and efficient procedure for saturation mutagenesis using mixed oligodeoxynucleotides. Gene. 1986;46(2-3):145–152. doi: 10.1016/0378-1119(86)90398-7. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J Virol. 1983 Dec;48(3):685–696. doi: 10.1128/jvi.48.3.685-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. T1 oligonucleotide maps of N-, B-, and B leads to NB-tropic murine leukemia viruses derived from BALB/c. J Virol. 1978 Apr;26(1):143–152. doi: 10.1128/jvi.26.1.143-152.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. L., Landon J. C., Pienta R. J., Kubicek M. T., Valerio M. G., Loeb W. F., Chopra H. C. Responses of infant rhesus monkeys to inoculation with Mason-Pfizer monkey virus materials. J Natl Cancer Inst. 1975 Mar;54(3):651–658. [PubMed] [Google Scholar]
- Fine D., Schochetman G. Type D primate retroviruses: a review. Cancer Res. 1978 Oct;38(10):3123–3139. [PubMed] [Google Scholar]
- Garry R. F. Extensive antigenic mimicry by retrovirus capsid proteins. AIDS Res Hum Retroviruses. 1990 Dec;6(12):1361–1362. doi: 10.1089/aid.1990.6.1361. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS. 1991 Jun;5(6):617–637. [PubMed] [Google Scholar]
- Gelderblom H. R., Hausmann E. H., Ozel M., Pauli G., Koch M. A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987 Jan;156(1):171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Lobel L. I. Mutants of murine leukemia viruses and retroviral replication. Biochim Biophys Acta. 1987 Jul 8;907(2):93–123. doi: 10.1016/0304-419x(87)90001-1. [DOI] [PubMed] [Google Scholar]
- Gorelick R. J., Nigida S. M., Jr, Bess J. W., Jr, Arthur L. O., Henderson L. E., Rein A. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J Virol. 1990 Jul;64(7):3207–3211. doi: 10.1128/jvi.64.7.3207-3211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Jelinek L., Whiting S., Barklis E. Transport and assembly of gag proteins into Moloney murine leukemia virus. J Virol. 1990 Nov;64(11):5306–5316. doi: 10.1128/jvi.64.11.5306-5316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R., Smythers G., Benveniste R. E., Oroszlan S. Purification and N-terminal amino acid sequence comparisons of structural proteins from retrovirus-D/Washington and Mason-Pfizer monkey virus. J Virol. 1985 Sep;55(3):778–787. doi: 10.1128/jvi.55.3.778-787.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. W., Schwartzberg P., Goff S. P. Point mutations in the P30 domain of the gag gene of Moloney murine leukemia virus. Virology. 1985 Apr 15;142(1):211–214. doi: 10.1016/0042-6822(85)90435-0. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J Virol. 1980 Jan;33(1):183–195. doi: 10.1128/jvi.33.1.183-195.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Blaug G., Hansen M., Barklis E. Assembly of gag-beta-galactosidase proteins into retrovirus particles. J Virol. 1990 May;64(5):2265–2279. doi: 10.1128/jvi.64.5.2265-2279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel L. I., Goff S. P. Construction of mutants of Moloney murine leukemia virus by suppressor-linker insertional mutagenesis: positions of viable insertion mutations. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4149–4153. doi: 10.1073/pnas.81.13.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx P. A., Maul D. H., Osborn K. G., Lerche N. W., Moody P., Lowenstine L. J., Henrickson R. V., Arthur L. O., Gilden R. V., Gravell M. Simian AIDS: isolation of a type D retrovirus and transmission of the disease. Science. 1984 Mar 9;223(4640):1083–1086. doi: 10.1126/science.6695196. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Méric C., Gouilloud E., Spahr P. F. Mutations in Rous sarcoma virus nucleocapsid protein p12 (NC): deletions of Cys-His boxes. J Virol. 1988 Sep;62(9):3328–3333. doi: 10.1128/jvi.62.9.3328-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patarca R., Haseltine W. A. A major retroviral core protein related to EPA and TIMP. 1985 Nov 28-Dec 4Nature. 318(6044):390–390. doi: 10.1038/318390a0. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Cappiello D., Wilkowski C., Vogt V. M. Chemical crosslinking of proteins in avian sarcoma and leukemia viruses. Virology. 1980 Apr 15;102(1):205–210. doi: 10.1016/0042-6822(80)90081-1. [DOI] [PubMed] [Google Scholar]
- Pettit S. C., Simsic J., Loeb D. D., Everitt L., Hutchison C. A., 3rd, Swanstrom R. Analysis of retroviral protease cleavage sites reveals two types of cleavage sites and the structural requirements of the P1 amino acid. J Biol Chem. 1991 Aug 5;266(22):14539–14547. [PubMed] [Google Scholar]
- Rhee S. S., Hui H. X., Hunter E. Preassembled capsids of type D retroviruses contain a signal sufficient for targeting specifically to the plasma membrane. J Virol. 1990 Aug;64(8):3844–3852. doi: 10.1128/jvi.64.8.3844-3852.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Amino acid substitutions within the matrix protein of type D retroviruses affect assembly, transport and membrane association of a capsid. EMBO J. 1991 Mar;10(3):535–546. doi: 10.1002/j.1460-2075.1991.tb07980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J Virol. 1987 Apr;61(4):1045–1053. doi: 10.1128/jvi.61.4.1045-1053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T. L., Devare S. G., Blennerhassett G. T., Stephenson J. R. Nonconditional replication mutants of type C and type D retroviruses defective in gag gene-coded polyprotein post-translational processing. Virology. 1978 Dec;91(2):352–363. doi: 10.1016/0042-6822(78)90383-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Gordon M. L., Goff S. P. Mutations in the gag gene of Moloney murine leukemia virus: effects on production of virions and reverse transcriptase. J Virol. 1984 Mar;49(3):918–924. doi: 10.1128/jvi.49.3.918-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Stromberg K., Benveniste R. E., Arthur L. O., Rabin H., Giddens W. E., Jr, Ochs H. D., Morton W. R., Tsai C. C. Characterization of exogenous type D retrovirus from a fibroma of a macaque with simian AIDS and fibromatosis. Science. 1984 Apr 20;224(4646):289–282. doi: 10.1126/science.6200929. [DOI] [PubMed] [Google Scholar]
- Stromberg K., Hurley N. E., Davis N. L., Rueckert R. R., Fleissner E. Structural studies of avian myeloblastosis virus: comparison of polypeptides in virion and core component by dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1974 Feb;13(2):513–528. doi: 10.1128/jvi.13.2.513-528.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto Y. A., Cardiff R. D., Lund J. K. The structure of the mouse mammary tumor virus: isolation and characterization of the core. Virology. 1977 Mar;77(1):135–148. doi: 10.1016/0042-6822(77)90413-5. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C. Form, function, and use of retroviral gag proteins. AIDS. 1991 Jun;5(6):639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Kiggans J. O., Yang D. M., Ou C. Y., Tennant R. W., Brown A., Bassin R. H. Synthesis and circularization of N- and B-tropic retroviral DNA Fv-1 permissive and restrictive mouse cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2994–2998. doi: 10.1073/pnas.77.5.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]