Abstract
The sperm acrosome reaction is a Ca2+-dependent exocytotic event that is triggered by adhesion to the mammalian egg’s zona pellucida. Previous studies using ion-selective fluorescent probes suggested a role of voltage-sensitive Ca2+ channels in acrosome reactions. Here, whole-cell patch clamp techniques are used to demonstrate the expression of functional T-type Ca2+ channels during mouse spermatogenesis. The germ cell T current is inhibited by antagonists of T-type channels (pimozide and amiloride) as well as by antagonists whose major site of action is the somatic cell L-type Ca2+ channel (1,4-dihydropyridines, arylalkylamines, benzothiazapines), as has also been reported for certain somatic cell T currents. In sperm, inhibition of T channels during gamete interaction inhibits zona pellucida-dependent Ca2+ elevations, as demonstrated by ion-selective fluorescent probes, and also inhibits acrosome reactions. These studies directly link sperm T-type Ca2+ channels to fertilization. In addition, the kinetics of channel inhibition by 1,4-dihydropyridines suggests a mechanism for the reported contraceptive effects of those compounds in human males.
The acrosome reaction, a Ca2+-dependent exocytotic event in sperm, is an obligatory early step in the fertilization process that must be completed prior to fusion with eggs. In mammals, it is triggered by contact with the egg’s extracellular matrix, or zona pellucida. The signal-transducing mechanisms that couple gamete adhesion to acrosome reactions play a central role in fertilization and also provide possible targets for contraceptive intervention (1).
Particular attention has focused on the control of sperm internal Ca2+ (Cai2+) during fertilization. Zona pellucida adhesion elevates sperm Cai2+, as reported by ion-selective fluorescent probes, yet the Ca2+ entry pathways have not been identified directly. In this regard, mammalian sperm have voltage-sensitive Ca2+ channels (2–4), and antagonists that act primarily at L-type Ca2+ channels inhibit both the zona pellucida-dependent Cai2+ elevation and the initiation of acrosome reactions (3, 5). Moreover, one class of L-type channel antagonists, the 1,4-dihydropyridines, have been reported to act as a human male contraceptive (6, 7). These observations suggest a role for a putative L-type channel of sperm during gamete interaction. Yet several features of the sperm Cai2+ regulatory pathway are inconsistent with the presence of L-type channels. (i) The selectivity of divalent metal cation block (Ni2+ > Cd2+) is the reverse of that anticipated at L channels. (ii) Bay K8644, an agonist of somatic cell L-type channels, fails to affect sperm Ca2+ channel function or to bind to sperm membranes. (iii) The sperm pathway is inhibited by L channel antagonists, including arylalkylamines, benzothiazapines, and 1,4-dihydropyridines, but at 10- to 100-fold higher concentrations than typically observed at somatic cell L channels (see discussion in refs. 3 and 5). The nature of the zona pellucida-activated Cai2+ entry pathway is thus unresolved.
Here, we apply patch clamp methods to assess directly the expression of functional Ca2+ channels during spermatogenesis. The role of specific channels in fertilization is determined by using Ca2+-selective fluorescent probes. These studies indicate that a T-type Ca2+ channel is present on male germ cells and can account for both the zona pellucida-induced Ca2+ influx during acrosome reactions and the reported antifertility effects of 1,4-dihydropyridines.
MATERIALS AND METHODS
Biological Preparations.
Spermatogenic cells and sperm were obtained from CD-1 mice by manual trituration of testicular slices (8) and from caudae epididymides, respectively. Methods for sperm capacitation in a Hepes-buffered medium, for sperm–zona pellucida adhesion assays, and for determination of acrosome reactions by Coomassie blue staining have been described (3, 9–12). The fraction of motile sperm was determined by visual examination. Zonae pellucidae were isolated by manual dissection from germinal vesicle-intact, follicular oocytes. Soluble extracts were prepared in 5 mM NaH2PO4 (pH 2.5) and adjusted to pH 7.4 prior to use (13).
Electrophysiological Methods.
Spermatogenic cells were immobilized on culture dishes coated with Cell-Tak (Collaborative Biomedical Products, Bedford, MA) and perfused with a bath solution containing (mM): NaCl (100), KCl (5), CaCl2 (10), MgCl2 (1), N(C2H5)4Cl (26), sodium lactate (6), Hepes (10, pH 7.4 with NaOH), and d-glucose (3.3).
Hard glass (Gardner #7052; 2–10 MΩ pipette resistance), Sylgard-coated pipettes were used to voltage clamp spermatogenic cells in the whole-cell configuration of the patch clamp method, as described by Hamill et al. (14). The pipette internal solution contained (mM): cesium glutamate (120), MgCl2 (5), N(C2H5)4Cl (20), Mg2ATP (3), CsEGTA (10), Hepes (10, pH 7.0 with CsOH), and d-glucose (5). Ca2+ currents were recorded using an Axopatch 1-D amplifier (Axon Instruments, Foster City, CA). Data were sampled at 10 kHz, filtered at 3 kHz, corrected for leakage and capacitance currents, and analyzed with biopatch (Biologic, Grenoble, France).
High-resistance seals form readily on spermatogenic cells but are difficult to establish on sperm. Cell geometry is important, as seals can be formed on sperm with flattened, spatulate-shaped heads (bovine, ovine) more frequently than on sperm with crescent-shaped heads (mouse, hamster, guinea pig). In addition, the mouse sperm plasma membrane is relatively noncompliant, possibly due to interactions with the cytoskeleton, and fractures during attempts to form high-resistance seals. As a result, patch clamp techniques were applied only to spermatogenic cells and not to sperm.
Cai2+ Determinations.
Sperm were incubated 0.25 hr with fura 2-AM (the acetoxymethyl ester) (1–3 μM; Molecular Probes) and immobilized on Cell-Tak-coated glass coverslips. Extracellular fura 2 and fura 2-AM were removed by perfusion, cells were placed on a heated microscope stage, and fluorescent images were acquired, stored, and analyzed, as described previously (5, 15, 16). Data are expressed as the relative Cai2+ concentration and related to the internal ion concentration, [Ca2+]i, by the relationship Cai2+ = [Ca2+]i/Kd = [(Rmax − R)/(R − Rmin)] × B, where R, Rmin, and Rmax are the emission ratios after sequential excitation at 340 and 380 nm in samples and in Ca2+-depleted and Ca2+-saturated solutions, respectively; B is a sensitivity factor; and Kd is the affinity of Ca2+·fura 2 complexes (17). Average cellular Cai2+ is derived by integration of local values. Rates (ΔCai2+/min) are the slopes of regression lines calculated for the linear portions of Cai2+ time courses.
RESULTS
Ca2+ Channel Characterization.
Whole-cell patch recordings of diploid (pachytene spermatocytes) and haploid (round and condensing spermatids) stages of the male mouse germ lineage reveal an inward Ca2+ current (4.85 ± 1.03 μA/cm2, n = 57) with an activation threshold at −60 mV and a peak current at −30 mV (Fig. 1 A and B), similar to that reported in rat spermatogenic cells (8).
Figure 1.
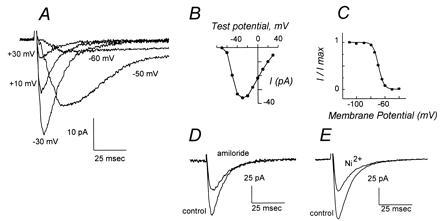
Ca2+ currents of mouse spermatogenic cells are mediated by low-voltage-activated T channels. (A) Montage of whole-cell Ca2+ current traces after depolarizations of a round spermatid to the indicated membrane potentials from a holding potential of −90 mV. A slowly inactivating component of ICa after depolarization to positive potentials, diagnostic of L currents, is not apparent. (B) Current–voltage relationship for peak current amplitude elicited by depolarizations from a holding potential of −90 mV. (C) Voltage dependence of steady-state inactivation of round spermatid Ca2+ current. (D) Inhibition of peak Ca2+ current amplitude by amiloride. Traces show current elicited by depolarizations from −80 mV to −20 mV in a cell prior to (control) and after addition of 200 μM amiloride. (E) Inhibition of peak Ca2+ current amplitude by Ni2+. Traces show current elicited by depolarizations from -80 mV to -20 mV in a cell prior to (control) and following addition of 50 μM Ni2+. Current scales are indicated and differ between panels A, D, and E.
The mouse germ cell Ca2+ current has the anticipated characteristics of somatic cell T-type channels. (i) The current activates at low depolarizations with a threshold that is typical for only the T-type channel and inconsistent with the presence of high-voltage-activated channels such as the L-, N-, or P/Q-type channels (Fig. 1B). (ii) The voltage range for inactivation is also characteristic of T-type channels (Fig. 1C; refs. 18–20). (iii) Current is inhibited by amiloride (IC50 ≈ 245 μM; Fig. 1D) and by pimozide (IC50 ≈ 0.47 μM; Fig. 2A), antagonists of somatic cell T channels (20, 21). In contrast, spermatogenic cell Ca2+ currents are not affected by ethosuximide (data not shown), an anticonvulsant that blocks only a restricted population of T channels (20, 22). (iv) The relative potency of metal cation inhibition of this current is Ni2+ (IC50 ≈ 34 μM; Fig. 1E) > Cd2+ (IC50 ≈ 285 μM; data not shown), similar to that observed at somatic cell T-type channels and the reverse of that found for L-type channels. (v) Inward current in spermatogenic cells is not enhanced when Ba2+ replaces Ca2+ as the charge carrier (ICa ≈ IBa). The biophysical and pharmacological properties of the spermatogenic cell Ca2+ current indicate that it is mediated by a T-type channel.
Figure 2.
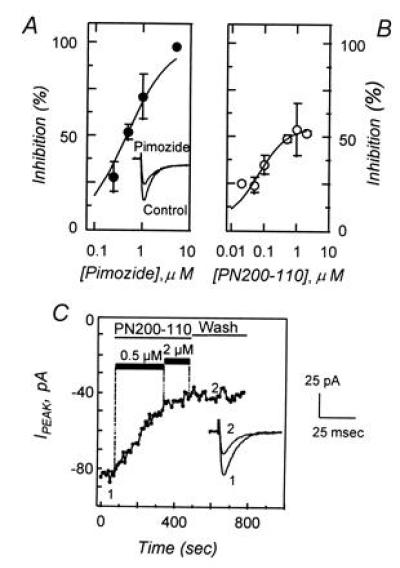
Low-voltage-activated Ca2+ currents are inhibited by pimozide and by PN200-110. (A) Dose–response relationship showing the effects of pimozide (•) on the peak Ca2+ current density after depolarization to −20 mV from a holding potential of −80 mV. Data represent the mean (±SEM) response of five to eight cells and was fit to the relationship D/Dmax = (V·[C])/(Ki + [C]), where D/Dmax is the proportion of current density remaining after pimozide treatment, [C] is pimozide concentration, V is the maximum inhibition, and Ki is the inhibitory constant. In this data set V and Ki were determined to be 100% and 467 nM, respectively. (Inset) Comparison of control Ca2+ current and currents after treatment with 1 μM pimozide. (B) Dose–response relationship showing the effects of PN200-110 (○) on the peak Ca2+ current density, determined under conditions similar to those in A, with derived values of V = 53% and Ki = 39 nM. Data are obtained from one to six cells. (C) Peak Ca2+ current during repetitive depolarizations to −20 mV from a holding potential of −80 mV. Depolarizations (100 msec) were provided at a frequency of 0.2 Hz. Control currents were recorded, PN200-110 was introduced by perfusion (0.5 and 2 μM; solid bars), and peak currents were recorded for several minutes. Recovery was followed for 5 min after removal of PN200-110. Representative Ca2+ current traces are illustrated before (point 1) and after (point 2) PN200-110 treatment. Scale bars for current traces are shown on the right.
Any channels that are required by sperm must be synthesized during spermatogenesis, since sperm are transcriptionally and translationally inactive. Previously, examination of sperm Cai2+ using ion-selective fluorescent probes suggested that zona pellucida contact activates a putative L-type channel (3). Yet inspection of spermatogenic cell Ca2+ currents does not reveal the presence of an L-type component (Fig. 1 A and B). L channels produce a high-voltage-activated, slowly inactivating current that would persist after inactivation of T channels during depolarization to positive membrane potentials. We attempted to detect L currents by recording Ba2+ currents in the presence of an L channel agonist, Bay K8644. Even though L channels conduct Ba2+ at 2- to 3-fold greater rates than Ca2+ (18, 20), we found no evidence for an L channel-mediated component of spermatogenic cell Ca2+ currents (data not shown).
Effects of L Channel Antagonists on Germ Cell T Channels.
The dual observations that L channel antagonists inhibit the zona pellucida-dependent elevation of sperm Cai2+ (3, 5, 23) and that germ cells lack a detectable L current led us to examine the effects of these antagonists on the T current. The spermatogenic cell T channel is inhibited by several classes of L channel antagonists. PN200-110, the high-affinity 1,4-dihydropyridine antagonist of somatic L channels (24), blocks approximately 50% of the spermatogenic cell T current (IC50 ≈ 39 nM; Fig. 2B). Inhibition is due to reduced peak Ca2+ current, without obvious effects on either activation or inactivation kinetics (Fig. 2C Inset). The effects of PN200-110 are not readily reversible, with no detectable recovery during 5-min wash periods (Fig. 2C). The spermatogenic cell T current is also inhibited by certain other L channel antagonists, including another 1,4-dihydropyridine (nifedipine), a benzothiazapine (diltiazem), and an arylalkylamine (verapamil; not shown). The inhibition of spermatogenic T-type channels by verapamil is reversible (50% recovery in ≈30 sec), unlike the effects of PN200-110. Certain other L channel ligands, such as Bay K8644, do not affect spermatogenic T channels (not shown). Thus, the spermatogenic cell T channel is similar to somatic cell T channels in its sensitivity to inhibition by some Ca2+ entry blockers that are generally believed to act as L channel antagonists, including the 1,4-dihydropyridines (25–27).
T Channel Activation During Sperm–Egg Interaction.
The ability of antagonists that principally act at somatic cell L channels to inhibit the spermatogenic cell T channel, coupled with the absence of detectable L channels in the germ cell lineage, suggests that the inhibitory effects of these agents on zona pellucida-dependent responses in sperm (3, 5, 23) may be mediated by T channels. This hypothesis was tested by examining the effects of specific T channel antagonists on sperm–egg interaction.
Zona pellucida glycoproteins promote sustained elevations of mouse sperm Cai2+ (Fig. 3A; ref. 27), as was also observed in bovine sperm (4, 5, 15). This response is inhibited by the same concentrations of pimozide and of amiloride (Fig. 3) that suppress spermatogenic cell T currents (Figs. 1D and 2A). The Cai2+ response is also inhibited by Ni2+ (44% ± 7% inhibition at 50 μM; n = 3) and by Cd2+ (59 ± 4% inhibition at 250 μM; n = 3) with potencies that correspond to the effects of these cations on the spermatogenic cell T current. These results suggest that the zona pellucida-induced Cai2+ elevation in mouse sperm is mediated by T channels.
Figure 3.
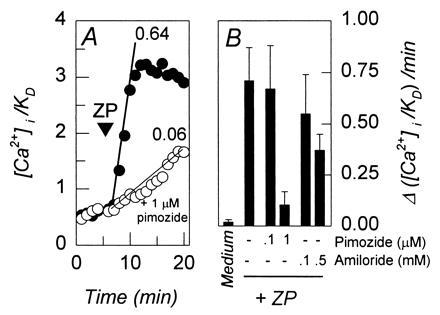
T channel antagonists inhibit the zona pellucida-dependent increase in Cai2+. [Ca2+]i/Kd values were determined from fluorescent emission of intracellular fura 2. (A) The effects of zona pellucida glycoproteins on [Ca2+]i/Kd in representative sperm that were incubated in control medium (•) or in medium containing 1 μM pimozide (10-min preincubation; ○). Solubilized zona pellucida glycoproteins were added (ZP, ▾; 50 μg/ml) and rates were determined by regression analysis of the linear portions of the response time courses. Slopes of regression lines are indicated. (B) Rates of [Ca2+]i/Kd responses obtained from experiments similar to that shown in A. Capacitated sperm were incubated for 10 min with indicated concentrations of pimozide or amiloride and then treated with solubilized zona pellucida glycoproteins (50 μg/ml). Control populations were treated with culture medium throughout. Data were obtained from four to six separate experiments, with 7–22 sperm observed per experiment. Average response rates were determined for each experiment and used to determine the indicated mean values (±SEM).
The effects of T channel antagonists on the acrosome reaction were determined by examining sperm during adhesion to intact zonae pellucidae. Mouse sperm bind to the zona pellucida in the absence of inhibitors, and >80% complete the acrosome reaction (Fig. 4 A and B), as described previously (28–30). Pimozide (1 μM) and amiloride (0.5 mM) had no apparent effect on sperm motility, on spontaneous rates of acrosome reaction, or on sperm–zona pellucida adhesion, but they greatly inhibited induction of acrosome reactions by zonae pellucidae after sperm adhesion (Fig. 4 A and C).
Figure 4.
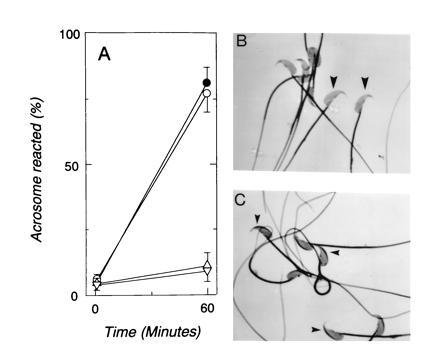
Effects of T channel antagonists on sperm acrosome reactions. (A) Occurrence (%) of acrosome reactions following sperm adhesion to zonae pellucidae for 1 and 60 min in control medium (•) or in medium supplemented with d-glucose (1 mM; ○), amiloride (500 μM; ▵), or pimozide (0.5 μM; ▿). Data repesent the mean (±SEM) of four experiments using 72–135 zonae pellucidae for each experimental condition and with 29–34 sperm bound per zona pellucida in all treatments. (B) Sperm bound to zona pellucida in control medium for 60 min. (×1000; zona pellucida staining subtracted.) Most sperm are acrosome reacted and do not exhibit dark staining in the apical head (large arrowheads). (C) Sperm bound to zona pellucida for 60 min in the presence of 0.5 μM pimozide. Most sperm fail to acrosome react, as indicated by a stained region in the apical head (small arrowheads).
T channel antagonists also inhibit the acrosome reaction that is promoted by soluble extracts of zonae pellucidae. Incubation with zona pellucida glycoproteins for 20 min increased the fraction of acrosome reacted sperm from 19% ± 5% to 62% ± 6% (n = 3; >200 sperm assayed per sample), as reported previously (28, 29). This is a specific effect of the zona pellucida, and only 23% ± 8% of sperm were acrosome reacted after incubation with control glycoproteins (50 μg/ml transferrin or fetuin). As shown in Table 1, pimozide and amiloride inhibit the zona pellucida-dependent acrosome reaction in a concentration-dependent fashion. Treatment with 1 μM pimozide or with 0.5 mM amiloride inhibited the secretory response to zonae pellucidae by 77% and by 72%, respectively, but had no significant effects on the incidence of acrosome reactions in populations treated with control glycoproteins. Yet sperm treated with T channel antagonists retain the ability to undergo acrosome reactions after addition of A23187 (5 μM; 20 min). This Ca2+-transporting ionophore induced acrosome reactions in 68% ± 7% and in 74% ± 9% of cells treated with pimozide and amiloride. A similar response was observed in control populations that had not been treated with T channel antagonists (77% ± 4% acrosome reacted; duplicate experiments, > 250 assayed per treatment). These control experiments demonstrate that drug treatment does not alter the ability of sperm to acrosome react after Cai2+ elevations and are consistent with the suggestion that these agents act by inhibiting a zona pellucida-induced Ca2+ influx.
Table 1.
Effects of T channel antagonists on the zona pellucida-induced acrosome reaction
| Treatment | Conc. | Acrosome reacted, %
|
|
|---|---|---|---|
| +Control | +ZP | ||
| Medium | — | 23 ± 8 | 62 ± 6* |
| Pimozide | 0.1 μM | 21 ± 3 | 49 ± 5* |
| 1.0 μM | 18 ± 6 | 27 ± 4† | |
| Amiloride | 0.1 mM | 17 ± 4 | 54 ± 7* |
| 0.5 mM | 22 ± 3 | 33 ± 4*† | |
Capacitated mouse sperm were incubated for 10 min in culture medium or in medium supplemented with the indicated T channel antagonists. The fraction of acrosome reacted sperm in culture medium-treated populations was 19% ± 5%, and this background value was not altered significantly by treatment with T channel antagonists (range of means for pimozide and amiloride, 16–20%). Sperm were incubated an additional 20 min after addition of control glycoproteins (+Control, 50 μg/ml transferrin or fetuin) or soluble extracts of zonae pellucidae (+ZP, 50 μg/ml) were added and acrosome reactions were assessed. The results are given as the mean (±SEM) of triplicate experiments, with triplicate samples obtained in each experiment and with 100–200 sperm assayed per sample.
Sperm treated with zonae pellucidae differ from sperm treated with control glycoproteins (P < 0.01).
Sperm treated with T channel antagonists differ from culture medium controls (P < 0.01).
DISCUSSION
The results of this study strongly suggest that only T-type Ca2+ channels are present in mouse sperm and are activated by the zona pellucida during fertilization. Previously, we demonstrated a role for sperm voltage-sensitive Ca2+ channels in the initiation of acrosome reaction (3, 5). In somatic cells there is a large family of voltage-sensitive Ca2+ channels, including the high-voltage-activated L-, N-, and P/Q-types of channels as well as the R- and T-type channels that are activated by smaller depolarizations (30–32). However, the subtype of Ca2+ channel that is present in sperm and activated by the zona pellucida had not previously been determined.
The functional expression of T channels during mammalian spermatogenesis was demonstrated previously in the rat, on the basis of biophysical characteristics of a Ca2+ current (8). This conclusion is confirmed here by demonstrating that the major voltage-sensitive Ca2+ channel of mouse spermatogenic cells has the biophysical and pharmacological characteristics anticipated of a T channel. The channel can be detected in the early meiotic stage of spermatogenesis and is retained thereafter. However, it is still not certain whether T channels are active during germ cell differentiation or are stored in the membrane for a primary function during fertilization.
This study provides what is to our knowledge the first identification of a sperm Ca2+ channel subtype that is activated by sperm contact with the zona pellucida. Ca2+ channel function was examined in sperm by using ion-selective fluorescent probes. The zona pellucida-activated channel of sperm was inhibited by several distinct structural classes of Ca2+ entry antagonists (3, 5). A major site of action of these compounds in somatic cells is the L-type Ca2+ channel (30, 32). Yet other features of the sperm mechanism were inconsistent with the presence of L channels, including the low potency for inhibition by L channel antagonists, the failure of Bay K8644 to modulate sperm Cai2+, and a selectivity for inhibition by divalent metal cations (3, 5).
Three aspects of the present study extend these earlier observations. First, the spermatogenic cell T channel accounts for all of the characteristics of zona pellucida-activated mechanism in sperm, including those that are inconsistent with the presence of an L channel. For example, similar concentrations of PN200-110 (0.039 and 0.07 μM), Ni2+ (34 and 50 μM), and Cd2+ (245 and 250–500 μM) are required to inhibit T channels in spermatogenic cells and zona pellucida-activated Ca2+ channel function by 50% (present study; ref. 3). Given that T currents are monitored by electrophysiological methods while sperm Ca2+ channels are assessed by using fluorescent probes, the agreement between these effective inhibitory doses is quite close. The T current also is similar to the zona pellucida-regulated Ca2+ channel in that neither is modulated by Bay K8644.
Second, the zona pellucida-dependent activation of sperm Ca2+ channels and the initiation of acrosome reactions are inhibited by pimozide and amiloride, antagonists that suppress somatic cell T currents (Figs. 3 and 4). These compounds also inhibit the spermatogenic cell Ca2+ current (Figs. 1 and 2). Pimozide and amiloride can discriminate between T channels and other classes of voltage-sensitive Ca2+ channels (19–21), thereby further associating the T channel with zona pellucida-dependent responses. Although these compounds also act in somatic cells at sites other than Ca2+ channels, such as the Na+/H+ exchange mechanism (33–35), these alternative targets do not appear to be present in mammalian sperm (16, 36). Third, we have confirmed that the T-type channel is the only voltage-sensitive Ca2+ channel that can be detected in mammalian spermatogenic cells (8). These observations strongly suggest that the spermatogenic cell T channel is retained by sperm after the completion of spermatogenesis and mediates the zona pellucida-dependent Ca2+ influx during initiation of acrosome reactions. It should be noted that the electrophysiological and pharmacological characteristics of the germ cell T channel are similar to those determined previously for these channels in somatic cells (18–21, 25–27).
T channels are present in a wide range of excitable cells, including neurons, skeletal muscle, cardiac muscle, smooth muscle, neuroendocrine cells, and endocrine cells (20, 32, 37, 38). Channel activity is typically associated with rhythmic burst activity, such as in the oscillations of thalamic reticular neurons (27) or in cardiac muscle, where T channels contribute to pacemaker current (26, 39). These channels also enhance synaptic transmission and lower threshold processes (27, 30). In contrast, T channels are not generally believed to participate in exocytosis, although some indirect evidence supports an undefined role in certain somatic cell secretory systems (refs. 40 and 41, but also see ref. 42). In this regard, the present study demonstrates that sperm T channel function is essential for acrosomal exocytosis.
The specific role of T channel-mediated Ca2+ influx in zona pellucida-dependent signal transduction has not been determined. Zona pellucida signals produce sustained elevations of sperm Cai2+ that are required for the acrosome reaction (4, 5, 15, 43). In contrast, T-type channels mediate small, transient Ca2+ fluxes as a consequence of their rapid inactivation (Fig. 1; refs. 18–20). While the Ca2+ buffering and efflux mechanisms of sperm are not well understood (44, 45), it is unlikely that such processes could permit T channels to produce sustained Cai2+ responses. Alternatively, a transient Ca2+ influx through zona pellucida-activated T channels may activate Ca2+-dependent Cai2+ elevation. In this regard, recent studies show that mammalian sperm contain sequestered Ca2+ pools, and thapsigargin-induced emptying of these pools results in acrosome reactions (46, 47). Moreover, inositol trisphosphate (IP3) receptors are present in acrosomal membranes, and Ca2+ efflux from internal pools of permeabilized sperm occurs through an IP3-sensitive pathway (47). Conductance through the IP3 receptor/Ca2+ release pathway is positively modulated by Cai2+ (48) and provides a likely amplification mechanism for Ca2+ entering sperm through any pathway, including through zona pellucida-activated T-type channels.
In this model T channels would generate a triggering pulse of Ca2+ that is necessary for sustained Cai2+ elevations and for acrosome reactions. Other zona pellucida-dependent responses, such as a transient elevation of internal pH (4, 15) and the production of IP3 (49), can act in concert with T currents to regulate Ca2+ release from acrosomal stores, although Ca2+ influx through T channels is essential for this mechanism (Figs. 3 and 4). This complex regulatory system is consistent with previous observations that sperm membrane potential and internal pH act synergistically to promote sustained elevations of Cai2+ (2–4, 15). This mechanism permits stringent control of exocytosis in sperm, which have a single secretory vesicle and must strictly coordinate exocytosis with egg contact (1).
These results may also account for the reported contraceptive action of 1,4-dihydropyridines in human males (6, 7). The principal somatic cell target of the 1,4-dihydropyridines is the L-type voltage-sensitive Ca2+ channel, where these drugs act as reversible antagonists and produce an antihypertensive effect. Therapeutic doses of 1,4-dihydropyridines maintained in males should not inhibit sperm Ca2+ channel function during fertilization if the drug target were acting at an L channel, since the several hours required in the female reproductive tract for sperm capacitation and transport to the site of fertilization (1) will permit dissociation of bound drug from sperm receptors. The observation that 1,4-dihydropyridines are potent and poorly reversible inhibitors of T channels in male germ cells (Fig. 2) provides a new mechanism of contraceptive action. In contrast, arylalkylamine and benzothiazapine compounds inhibit spermatogenic T currents reversibly and provide strategies for dissecting antihypertensive and contraceptive effects.
Note Added in Proof.
While this manuscript was in review, we became aware of the recent study of Lievano et al. (50), in which they report the presence of a 1,4-dihydropyridine-sensitive T-type Ca2+ channel on mouse spermatogenic cells.
Acknowledgments
We give special thanks to Qin Chen, Michelle Gonzales, Eric Reese, and Esther Valdez for technical assistance and to Vince Coccia for help with illustrations. Our work was supported by grants from the Philippe Foundation, the Bushrod H. Campbell and Adah F. Hall Charity Fund (C.A.), the Whitaker Foundation (R.A.C.), and the National Institutes of Health (R.A.C., HD27244; H.M.F., HD32177).
Footnotes
Abbreviation: IP3, inositol trisphosphate.
References
- 1.Yanagimachi R. In: The Physiology of Reproduction. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 189–317. [Google Scholar]
- 2.Babcock D F, Pfeiffer D R. J Biol Chem. 1987;262:15041–15047. [PubMed] [Google Scholar]
- 3.Florman H M, Corron M E, Kim T D-H, Babcock D F. Dev Biol. 1992;152:304–314. doi: 10.1016/0012-1606(92)90137-6. [DOI] [PubMed] [Google Scholar]
- 4.Arnoult C, Zeng Y, Florman H M. J Cell Biol. 1996;134:637–645. doi: 10.1083/jcb.134.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florman H M. Dev Biol. 1994;165:152–164. doi: 10.1006/dbio.1994.1242. [DOI] [PubMed] [Google Scholar]
- 6.Benoff S, Cooper G W, Hurley I, Mandel F S, Rosenfeld D L, Scholl G M, Gilbert B P, Hershlag A. Fertil Steril. 1994;62:606–617. [PubMed] [Google Scholar]
- 7.Hershlag A, Cooper G W, Benoff S. Hum Reprod. 1995;10:599–606. doi: 10.1093/oxfordjournals.humrep.a135996. [DOI] [PubMed] [Google Scholar]
- 8.Hagiwara S, Kawa K. J Physiol (London) 1984;356:135–149. doi: 10.1113/jphysiol.1984.sp015457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florman H M, Bechtol K B, Wassarman P M. Dev Biol. 1984;106:243–255. doi: 10.1016/0012-1606(84)90079-4. [DOI] [PubMed] [Google Scholar]
- 10.Florman H M, Wassarman P M. Cell. 1985;41:313–324. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller C C, Bleil J D, Kinloch R A, Wassarman P M. Dev Biol. 1990;137:276–286. doi: 10.1016/0012-1606(90)90254-g. [DOI] [PubMed] [Google Scholar]
- 12.Thaler C D, Cardullo R A. Biochemistry. 1995;34:7788–7795. doi: 10.1021/bi00024a002. [DOI] [PubMed] [Google Scholar]
- 13.Bleil J D, Wassarman P M. Dev Biol. 1980;76:185–202. doi: 10.1016/0012-1606(80)90371-1. [DOI] [PubMed] [Google Scholar]
- 14.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 15.Florman H M, Tombes R M, First N L, Babcock D F. Dev Biol. 1989;135:133–146. doi: 10.1016/0012-1606(89)90164-4. [DOI] [PubMed] [Google Scholar]
- 16.Zeng Y, Oberdorf J A, Florman H M. Dev Biol. 1996;173:510–520. doi: 10.1006/dbio.1996.0044. [DOI] [PubMed] [Google Scholar]
- 17.Grynkiewicz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 18.Fox A P, Nowycky M C, Tsien R W. J Physiol (London) 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsien R W, Lipscombe D, Madison D V, Bley K R, Fox A P. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 20.Herrington J C, Lingle J. J Neurophysiol. 1992;68:213–232. doi: 10.1152/jn.1992.68.1.213. [DOI] [PubMed] [Google Scholar]
- 21.Tang C-M, Presser F, Morad M. Science. 1988;240:213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- 22.Coulter D A, Huguenard J R, Prince D A. Ann Neurol. 1989;25:582–593. doi: 10.1002/ana.410250610. [DOI] [PubMed] [Google Scholar]
- 23.Clark E N, Corron M E, Florman H M. J Biol Chem. 1993;268:5309–5316. [PubMed] [Google Scholar]
- 24.Janis R A, Silver P J, Triggle D J. Adv Drug Res. 1987;116:309–591. [Google Scholar]
- 25.Akaike N, Kostyuk P G, Osipchuk Y V. J Physiol (London) 1989;412:181–195. doi: 10.1113/jphysiol.1989.sp017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald T F, Pelzer S, Trautwein W, Pelzer D J. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 27.Huguenard J R. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 28.Florman H M, Storey B T. Dev Biol. 1982;91:121–130. doi: 10.1016/0012-1606(82)90015-x. [DOI] [PubMed] [Google Scholar]
- 29.Bleil J D, Wassarman P M. Dev Biol. 1983;95:317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- 30.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 31.Clapham D E. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 32.Tsien R W, Lipscombe D, Madison D, Bley K, Fox A. Trends Neurosci. 1995;18:52–54. [PubMed] [Google Scholar]
- 33.Kleyman T R, Cragoe E J. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- 34.Enyeart J J, Biagi B A, Day R N, Sheu S-S, Maurer R A. J Biol Chem. 1990;265:16373–16379. [PubMed] [Google Scholar]
- 35.Hamill O P, Lane J W, McBride D W. Trends Pharmacol Sci. 1992;13:373–376. doi: 10.1016/0165-6147(92)90115-m. [DOI] [PubMed] [Google Scholar]
- 36.Babcock D F, Rufo G A, Lardy H A. Proc Natl Acad Sci USA. 1983;80:1327–1331. doi: 10.1073/pnas.80.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bean B P. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- 38.Tsien R W, Tsien R Y. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- 39.Hagiwara N, Irisawa H, Kameyama M. J Physiol (London) 1988;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enyeart J J, Mlinar B, Enyeart J A. Mol Endocrinol. 1993;7:1031–1040. doi: 10.1210/mend.7.8.8232302. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy R T, Isales C, Rasmussen H. Proc Natl Acad Sci USA. 1993;90:3260–3264. doi: 10.1073/pnas.90.8.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coyne M D, Pinkney L. Endocrinology. 1991;129:263–269. doi: 10.1210/endo-129-1-263. [DOI] [PubMed] [Google Scholar]
- 43.Bailey J L, Storey B T. Mol Reprod Dev. 1994;39:297–308. doi: 10.1002/mrd.1080390307. [DOI] [PubMed] [Google Scholar]
- 44.Noland T D, Olson G E, Garbers D L. Biol Reprod. 1983;29:987–998. doi: 10.1095/biolreprod29.4.987. [DOI] [PubMed] [Google Scholar]
- 45.Meizel S. Biol Rev. 1984;59:125–157. doi: 10.1111/j.1469-185x.1984.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 46.Blackmore P F. Cell Calcium. 1993;14:53–60. doi: 10.1016/0143-4160(93)90018-2. [DOI] [PubMed] [Google Scholar]
- 47.Walensky L D, Snyder S H. J Cell Biol. 1995;130:857–869. doi: 10.1083/jcb.130.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bezprozvanny I, Watras J, Ehrlich B E. Nature (London) 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 49.Tomes C N, McMaster C R, Saling P M. Mol Reprod Dev. 1996;43:196–204. doi: 10.1002/(SICI)1098-2795(199602)43:2<196::AID-MRD9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 50.Lievano A, Santi C M, Serrano J, Trevino C L, Bellve A R, Hernandez-Cruz A, Darszon A. FEBS Lett. 1996;388:150–154. doi: 10.1016/0014-5793(96)00515-7. [DOI] [PubMed] [Google Scholar]


