Abstract
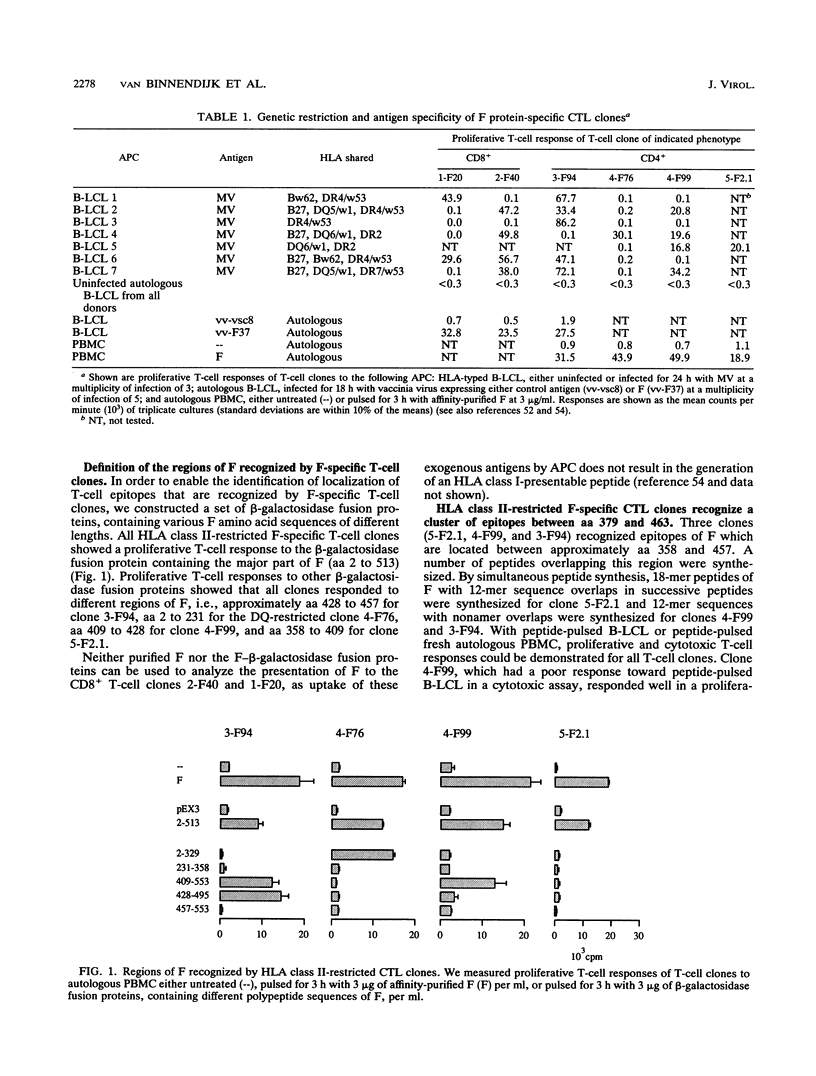
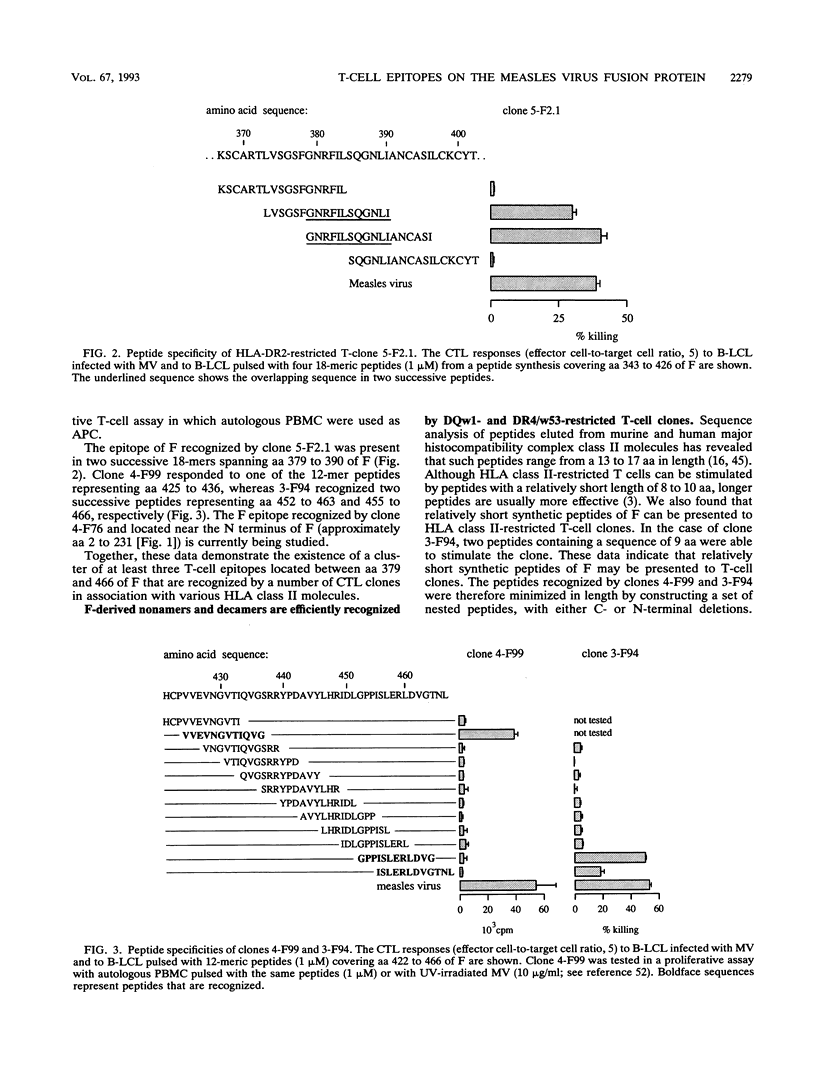
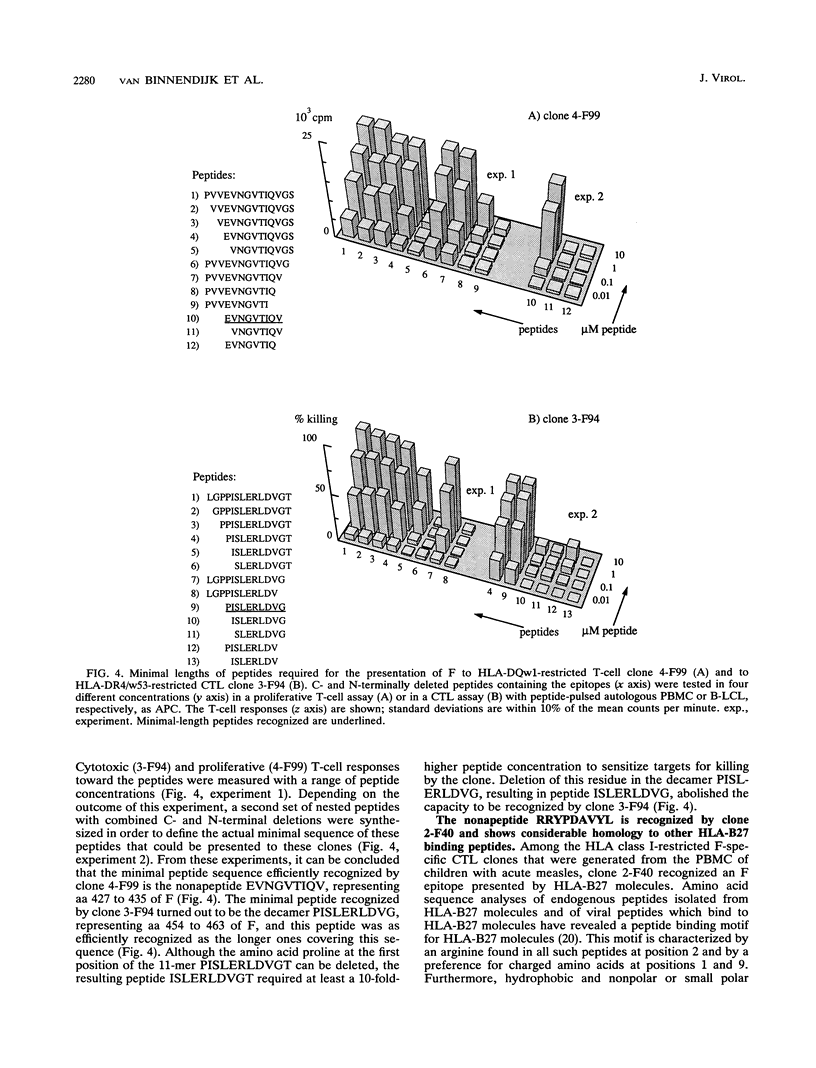
The transmembrane fusion (F) glycoprotein of measles virus is an important target antigen of human HLA class I- and class II-restricted cytotoxic T lymphocytes (CTL). Genetically engineered F proteins and nested sets of synthetic peptides spanning the F protein were used to determine sequences of F recognized by a number of F-specific CTL clones. Combined N- and C-terminal deletions of the respective peptides revealed that human HLA class I and HLA class II-restricted CTL efficiently recognize nonapeptides or decapeptides representing epitopes of F. Three distinct sequences recognized by three different HLA class II (DQw1, DR2, and DR4/w53)-restricted CTL clones appear to cluster between amino acids 379 and 466 of F, thus defining an important T-cell epitope area of F. Within this same region, a nonamer peptide of F was found to be recognized by an HLA-B27-restricted CTL clone, as expected on the basis of the structural homology between this peptide and other known HLA-B27 binding peptides.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braciale T. J., Braciale V. L. Antigen presentation: structural themes and functional variations. Immunol Today. 1991 Apr;12(4):124–129. doi: 10.1016/0167-5699(91)90096-C. [DOI] [PubMed] [Google Scholar]
- Cease K. B., Berkower I., York-Jolley J., Berzofsky J. A. T cell clones specific for an amphipathic alpha-helical region of sperm whale myoglobin show differing fine specificities for synthetic peptides. A multiview/single structure interpretation of immunodominance. J Exp Med. 1986 Nov 1;164(5):1779–1784. doi: 10.1084/jem.164.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. T., Markowitz L. E., Albrecht P., Stewart J. A., Mofenson L. M., Preblud S. R., Orenstein W. A. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990 Nov;162(5):1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- Crisanti A., Müller H. M., Hilbich C., Sinigaglia F., Matile H., McKay M., Scaife J., Beyreuther K., Bujard H. Epitopes recognized by human T cells map within the conserved part of the GP190 of P. falciparum. Science. 1988 Jun 3;240(4857):1324–1326. doi: 10.1126/science.2453924. [DOI] [PubMed] [Google Scholar]
- Drillien R., Spehner D., Kirn A., Giraudon P., Buckland R., Wild F., Lecocq J. P. Protection of mice from fatal measles encephalitis by vaccination with vaccinia virus recombinants encoding either the hemagglutinin or the fusion protein. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1252–1256. doi: 10.1073/pnas.85.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields G. B., Noble R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990 Mar;35(3):161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Giraudon P., Wild T. F. Correlation between epitopes on hemagglutinin of measles virus and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination inhibiting activity. Virology. 1985 Jul 15;144(1):46–58. doi: 10.1016/0042-6822(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Heeg K., Kuon W., Wagner H. Vaccination of class I major histocompatibility complex (MHC)-restricted murine CD8+ cytotoxic T lymphocytes towards soluble antigens: immunostimulating-ovalbumin complexes enter the class I MHC-restricted antigen pathway and allow sensitization against the immunodominant peptide. Eur J Immunol. 1991 Jun;21(6):1523–1527. doi: 10.1002/eji.1830210628. [DOI] [PubMed] [Google Scholar]
- Hill C. M., Hayball J. D., Allison A. A., Rothbard J. B. Conformational and structural characteristics of peptides binding to HLA-DR molecules. J Immunol. 1991 Jul 1;147(1):189–197. [PubMed] [Google Scholar]
- Huet S., Nixon D. F., Rothbard J. B., Townsend A., Ellis S. A., McMichael A. J. Structural homologies between two HLA B27-restricted peptides suggest residues important for interaction with HLA B27. Int Immunol. 1990;2(4):311–316. doi: 10.1093/intimm/2.4.311. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Richert J. R., Biddison W. E., Satinsky A., Hartzman R. J., McFarland H. F. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol. 1984 Aug;133(2):754–757. [PubMed] [Google Scholar]
- Jacobson S., Rose J. W., Flerlage M. L., McFarlin D. E., McFarland H. F. Induction of measles virus-specific human cytotoxic T cells by purified measles virus nucleocapsid and hemagglutinin polypeptides. Viral Immunol. 1987;1(3):153–162. doi: 10.1089/vim.1987.1.153. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Sekaly R. P., Jacobson C. L., McFarland H. F., Long E. O. HLA class II-restricted presentation of cytoplasmic measles virus antigens to cytotoxic T cells. J Virol. 1989 Apr;63(4):1756–1762. doi: 10.1128/jvi.63.4.1756-1762.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Kreth H. W., ter Meulen V., Eckert G. Demonstration of HLA restricted killer cells in patients with acute measles. Med Microbiol Immunol. 1979 Jan 24;165(4):203–214. doi: 10.1007/BF02152920. [DOI] [PubMed] [Google Scholar]
- Kropshofer H., Max H., Müller C. A., Hesse F., Stevanovic S., Jung G., Kalbacher H. Self-peptide released from class II HLA-DR1 exhibits a hydrophobic two-residue contact motif. J Exp Med. 1992 Jun 1;175(6):1799–1803. doi: 10.1084/jem.175.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C. J., Biddison W. E., Nelson D. L., Shaw S. Killing of measles virus-infected cells by human cytotoxic T cells. Infect Immun. 1982 Oct;38(1):226–232. doi: 10.1128/iai.38.1.226-232.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvoisin E., Wild F. Contribution of measles virus fusion protein in protective immunity: anti-F monoclonal antibodies neutralize virus infectivity and protect mice against challenge. J Virol. 1990 Oct;64(10):5160–5162. doi: 10.1128/jvi.64.10.5160-5162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz L. E., Sepulveda J., Diaz-Ortega J. L., Valdespino J. L., Albrecht P., Zell E. R., Stewart J., Zarate M. L., Bernier R. H. Immunization of six-month-old infants with different doses of Edmonston-Zagreb and Schwarz measles vaccines. N Engl J Med. 1990 Mar 1;322(9):580–587. doi: 10.1056/NEJM199003013220903. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hodgdon J. C., Cooper D. A., Hussey R. E., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Human cytotoxic T cell clones directed at autologous virus-transformed targets: further evidence for linkage of genetic restriction to T4 and T8 surface glycoproteins. J Immunol. 1983 Jul;131(1):186–190. [PubMed] [Google Scholar]
- Morein B., Sundquist B., Höglund S., Dalsgaard K., Osterhaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. 1984 Mar 29-Apr 4Nature. 308(5958):457–460. doi: 10.1038/308457a0. [DOI] [PubMed] [Google Scholar]
- Morgan A. J., Finerty S., Lovgren K., Scullion F. T., Morein B. Prevention of Epstein-Barr (EB) virus-induced lymphoma in cottontop tamarins by vaccination with the EB virus envelope glycoprotein gp340 incorporated into immune-stimulating complexes. J Gen Virol. 1988 Aug;69(Pt 8):2093–2096. doi: 10.1099/0022-1317-69-8-2093. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Donachie A. M., Reid G., Jarrett O. Immune-stimulating complexes containing Quil A and protein antigen prime class I MHC-restricted T lymphocytes in vivo and are immunogenic by the oral route. Immunology. 1991 Mar;72(3):317–322. [PMC free article] [PubMed] [Google Scholar]
- Nixon D. F., Townsend A. R., Elvin J. G., Rizza C. R., Gallwey J., McMichael A. J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988 Dec 1;336(6198):484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- Norrby E., Enders-Ruckle G., Meulen V. Differences in the appearance of antibodies to structural components of measles virus after immunization with inactivated and live virus. J Infect Dis. 1975 Sep;132(3):262–269. doi: 10.1093/infdis/132.3.262. [DOI] [PubMed] [Google Scholar]
- Norrby E., Gollmar Y. Appearance and persistence of antibodies against different virus components after regular measles infections. Infect Immun. 1972 Sep;6(3):240–247. doi: 10.1128/iai.6.3.240-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E. Occurrence of antibodies against envelope components after immunization with formalin inactivated and live measles vaccine. J Biol Stand. 1975;3(4):375–380. doi: 10.1016/0092-1157(75)90062-1. [DOI] [PubMed] [Google Scholar]
- Norrby E., Orvell C., Vandvik B., Cherry J. D. Antibodies against measles virus polypeptides in different disease conditions. Infect Immun. 1981 Dec;34(3):718–724. doi: 10.1128/iai.34.3.718-724.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan D., Arrhenius T., Sidney J., Del Guercio M. F., Albertson M., Wall M., Oseroff C., Southwood S., Colón S. M., Gaeta F. C. On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol. 1991 Oct 15;147(8):2663–2669. [PubMed] [Google Scholar]
- O'Sullivan D., Sidney J., Del Guercio M. F., Colón S. M., Sette A. Truncation analysis of several DR binding epitopes. J Immunol. 1991 Feb 15;146(4):1240–1246. [PubMed] [Google Scholar]
- Osterhaus A., Weijer K., Uytdehaag F., Jarrett O., Sundquist B., Morein B. Induction of protective immune response in cats by vaccination with feline leukemia virus iscom. J Immunol. 1985 Jul;135(1):591–596. [PubMed] [Google Scholar]
- Partidos C. D., Steward M. W. Prediction and identification of a T cell epitope in the fusion protein of measles virus immunodominant in mice and humans. J Gen Virol. 1990 Sep;71(Pt 9):2099–2105. doi: 10.1099/0022-1317-71-9-2099. [DOI] [PubMed] [Google Scholar]
- Richert J. R., Rose J. W., Reuben-Burnside C., Kearns M. C., Jacobson S., Mingioli E. S., Hartzman R. J., McFarland H. F., McFarlin D. E. Polypeptide specificities of measles virus-reactive T cell lines and clones derived from a patient with multiple sclerosis. J Immunol. 1986 Oct 1;137(7):2190–2194. [PubMed] [Google Scholar]
- Rose J. W., Bellini W. J., McFarlin D. E., McFarland H. F. Human cellular immune response to measles virus polypeptides. J Virol. 1984 Mar;49(3):988–991. doi: 10.1128/jvi.49.3.988-991.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Sato T. A., Kohama T., Sugiura A. Protective role of human antibody to the fusion protein of measles virus. Microbiol Immunol. 1989;33(7):601–607. doi: 10.1111/j.1348-0421.1989.tb02010.x. [DOI] [PubMed] [Google Scholar]
- Schmid D. S. The human MHC-restricted cellular response to herpes simplex virus type 1 is mediated by CD4+, CD8- T cells and is restricted to the DR region of the MHC complex. J Immunol. 1988 May 15;140(10):3610–3616. [PubMed] [Google Scholar]
- Sethi K. K., Stroehmann I., Brandis H. Generation of cytolytic T-cell cultures displaying measles virus specificity and human histocompatibility leukocyte antigen restriction. Infect Immun. 1982 May;36(2):657–661. doi: 10.1128/iai.36.2.657-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Takeshita T., Morein B., Putney S., Germain R. N., Berzofsky J. A. Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature. 1990 Apr 26;344(6269):873–875. doi: 10.1038/344873a0. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Trudel M., Boulay G., Séguin C., Nadon F., Lussier G. Control of infectious bovine rhinotracheitis in calves with a BHV-1 subunit-ISCOM vaccine. Vaccine. 1988 Dec;6(6):525–529. doi: 10.1016/0264-410x(88)90105-3. [DOI] [PubMed] [Google Scholar]
- Varsanyi T. M., Morein B., Löve A., Norrby E. Protection against lethal measles virus infection in mice by immune-stimulating complexes containing the hemagglutinin or fusion protein. J Virol. 1987 Dec;61(12):3896–3901. doi: 10.1128/jvi.61.12.3896-3901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser I. K., vd Bildt M. W., Brugge H. N., Reijnders P. J., Vedder E. J., Kuiper J., de Vries P., Groen J., Walvoort H. C., UytdeHaag F. G. Vaccination of harbour seals (Phoca vitulina) against phocid distemper with two different inactivated canine distemper virus (CDV) vaccines. Vaccine. 1989 Dec;7(6):521–526. doi: 10.1016/0264-410x(89)90276-4. [DOI] [PubMed] [Google Scholar]
- Wiertz E. J., van Gaans-van den Brink J. A., Gausepohl H., Prochnicka-Chalufour A., Hoogerhout P., Poolman J. T. Identification of T cell epitopes occurring in a meningococcal class 1 outer membrane protein using overlapping peptides assembled with simultaneous multiple peptide synthesis. J Exp Med. 1992 Jul 1;176(1):79–88. doi: 10.1084/jem.176.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild F., Giraudon P., Spehner D., Drillien R., Lecocq J. P. Fowlpox virus recombinant encoding the measles virus fusion protein: protection of mice against fatal measles encephalitis. Vaccine. 1990 Oct;8(5):441–442. doi: 10.1016/0264-410x(90)90243-f. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984 Jul;133(1):422–427. [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. The binary logic of antigen processing and presentation to T cells. Cell. 1990 Jul 27;62(2):203–206. doi: 10.1016/0092-8674(90)90356-j. [DOI] [PubMed] [Google Scholar]
- de Vries P., Van Binnendijk R. S., Van der Marel P., Beuvery E. C., Van Wezel A. L., Voorma H. O., Uytedehaag F. G., Osterhaus A. D. Inhibition of measles virus-induced cell-cell fusion with a monoclonal antibody directed against the haemagglutinin. Viral Immunol. 1987 Spring;1(1):25–34. doi: 10.1089/vim.1987.1.25. [DOI] [PubMed] [Google Scholar]
- de Vries P., Versteeg-van Oosten J. P., Visser I. K., van Binnendijk R. S., Langeveld S. A., Osterhaus A. D., Uytdehaag F. G. Measles virus-specific murine T cell clones: characterization of fine specificity and function. J Immunol. 1989 Apr 15;142(8):2841–2846. [PubMed] [Google Scholar]
- de Vries P., van Binnendijk R. S., van der Marel P., van Wezel A. L., Voorma H. O., Sundquist B., Uytdehaag F. G., Osterhaus A. D. Measles virus fusion protein presented in an immune-stimulating complex (iscom) induces haemolysis-inhibiting and fusion-inhibiting antibodies, virus-specific T cells and protection in mice. J Gen Virol. 1988 Mar;69(Pt 3):549–559. doi: 10.1099/0022-1317-69-3-549. [DOI] [PubMed] [Google Scholar]
- van Binnendijk R. S., Poelen M. C., Kuijpers K. C., Osterhaus A. D., Uytdehaag F. G. The predominance of CD8+ T cells after infection with measles virus suggests a role for CD8+ class I MHC-restricted cytotoxic T lymphocytes (CTL) in recovery from measles. Clonal analyses of human CD8+ class I MHC-restricted CTL. J Immunol. 1990 Mar 15;144(6):2394–2399. [PubMed] [Google Scholar]
- van Binnendijk R. S., Poelen M. C., de Vries P., Voorma H. O., Osterhaus A. D., Uytdehaag F. G. Measles virus-specific human T cell clones. Characterization of specificity and function of CD4+ helper/cytotoxic and CD8+ cytotoxic T cell clones. J Immunol. 1989 Apr 15;142(8):2847–2854. [PubMed] [Google Scholar]
- van Binnendijk R. S., van Baalen C. A., Poelen M. C., de Vries P., Boes J., Cerundolo V., Osterhaus A. D., UytdeHaag F. G. Measles virus transmembrane fusion protein synthesized de novo or presented in immunostimulating complexes is endogenously processed for HLA class I- and class II-restricted cytotoxic T cell recognition. J Exp Med. 1992 Jul 1;176(1):119–128. doi: 10.1084/jem.176.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]



