Abstract
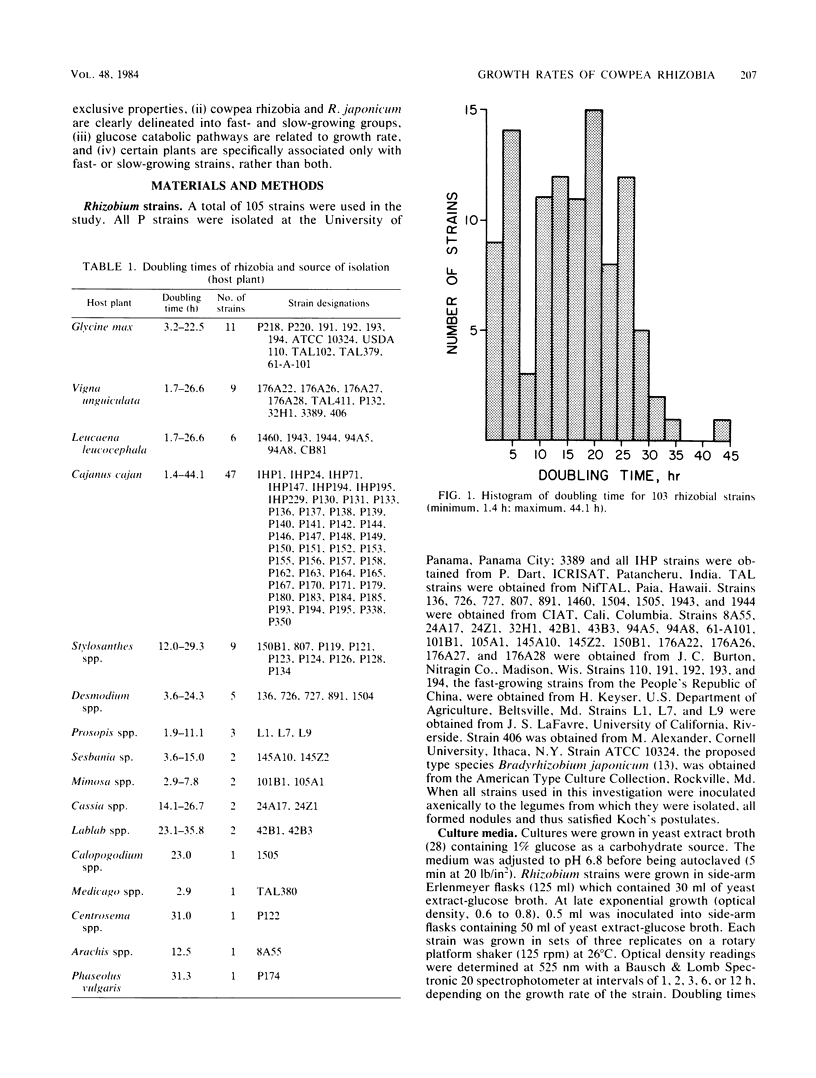
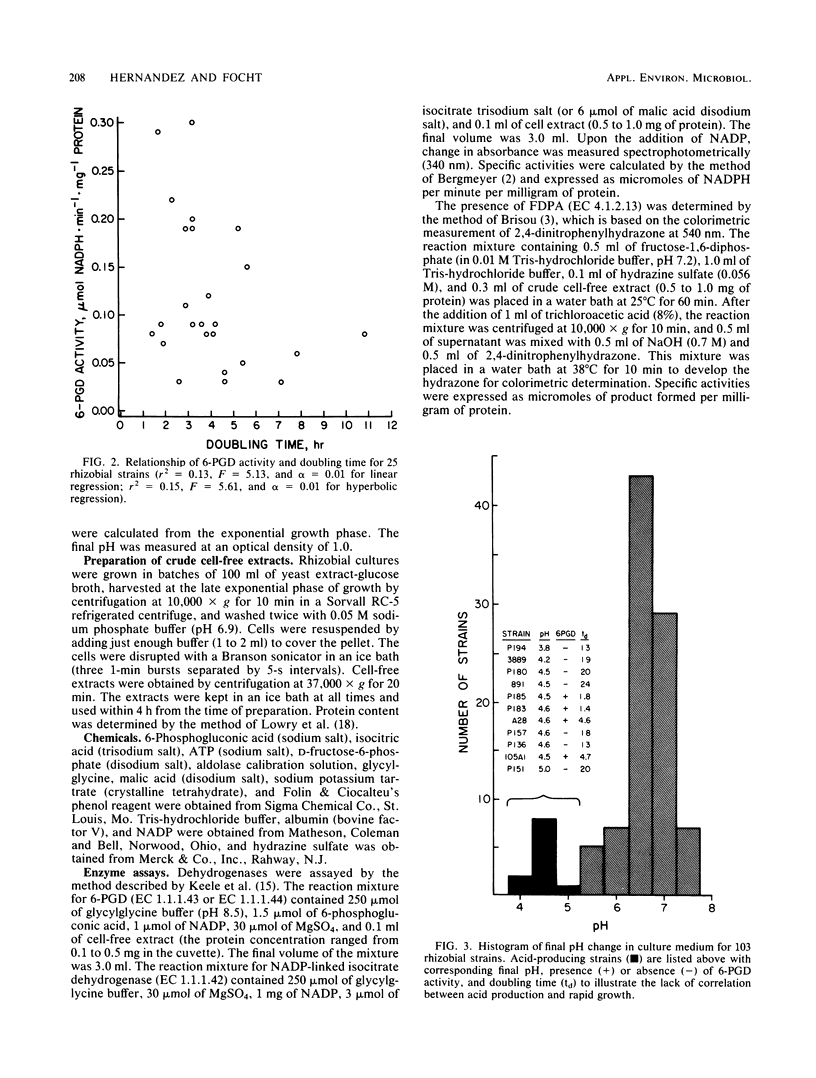
A total of 103 rhizobial strains representing the cowpea miscellany and Rhizobium japonicum were studied with regard to growth rate, glucose metabolic pathways, and pH change in culture medium. Doubling times ranged from 1.4 ± 0.04 to 44.1 ± 5.2 h; although two populations of “fast-growing” and “slow-growing” rhizobia were noted, they overlapped and were not distinctly separated. Twenty-four strains which had doubling times of less than 8 h all showed NADP-linked 6-phosphogluconate dehydrogenase (6-PGD) activity, whereas only one slow-growing strain (doubling time, 10.8 ± 0.9 h) of all those tested showed 6-PGD activity. Doubling times among fast growers could not be explained solely by the presence or absence of 6-PGD activity (r2 = 0.14) because the tricarboxylic acid cycle and the Emden-Meyerhoff-Parnas pathway were operative in both 6-PGD-positive and 6-PGD-negative strains. Growth rate and pH change were unrelated to each other. Fast- or slow-growing strains were not associated with any particular legume species or group of species from which they were originally isolated, with the exception of Stylosanthes spp., all nine isolates of which were slow growers. We conclude that 6-PGD activity is a more distinctive characteristic among physiologically different groups of rhizobia than doubling times and that characterization of the cowpea rhizobia as slow-growing alkali producers is an invalid concept.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin I. L., Fred E. B. NOMENCLATURE OF THE ROOT-NODULE BACTERIA OF THE LEGUMINOSAE. J Bacteriol. 1929 Feb;17(2):141–150. doi: 10.1128/jb.17.2.141-150.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley J., Rassel A. DNA base composition, flagellation and taxonomy of the genus Rhizobium. J Gen Microbiol. 1965 Oct;41(1):85–91. doi: 10.1099/00221287-41-1-85. [DOI] [PubMed] [Google Scholar]
- Dreyfus B. L., Dommergues Y. R. Nodulation of acacia species by fast- and slow-growing tropical strains of Rhizobium. Appl Environ Microbiol. 1981 Jan;41(1):97–99. doi: 10.1128/aem.41.1.97-99.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZNELSON H., ZAGALLO A. C. Metabolism of rhizobia in relation to effectiveness. Can J Microbiol. 1957 Oct;3(6):879–884. doi: 10.1139/m57-097. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Glucose catabolism in Rhizobium japonicum. J Bacteriol. 1969 Mar;97(3):1184–1191. doi: 10.1128/jb.97.3.1184-1191.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Bohlool B. B., Hu T. S., Weber D. F. Fast-growing rhizobia isolated from root nodules of soybean. Science. 1982 Mar 26;215(4540):1631–1632. doi: 10.1126/science.215.4540.1631. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martínez-De Drets G., Arias A. Enzymatic basis for differentiation of Rhizobium into fast- and slow-growing groups. J Bacteriol. 1972 Jan;109(1):467–470. doi: 10.1128/jb.109.1.467-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett M. L., Colwell R. R. Adansonian analysis of the Rhizobiaceae. J Gen Microbiol. 1968 Apr;51(2):245–266. doi: 10.1099/00221287-51-2-245. [DOI] [PubMed] [Google Scholar]
- Mulongoy K., Elkan G. H. The role of 6-phosphogluconate dehydrogenase in Rhizobium. Can J Microbiol. 1977 Sep;23(9):1293–1298. doi: 10.1139/m77-193. [DOI] [PubMed] [Google Scholar]
- Skotnicki M. L., Rolfe B. G. Differential stimulation and inhibition of growth of Rhizobium trifolii strain T1 and other Rhizobium species by various carbon sources. Microbios. 1977;20(79):15–28. [PubMed] [Google Scholar]
- Zablotowicz R. M., Focht D. D. Physiological Characteristics of Cowpea Rhizobia: Evaluation of Symbiotic Efficiency in Vigna unguiculata. Appl Environ Microbiol. 1981 Mar;41(3):679–685. doi: 10.1128/aem.41.3.679-685.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



