Abstract
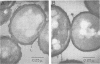
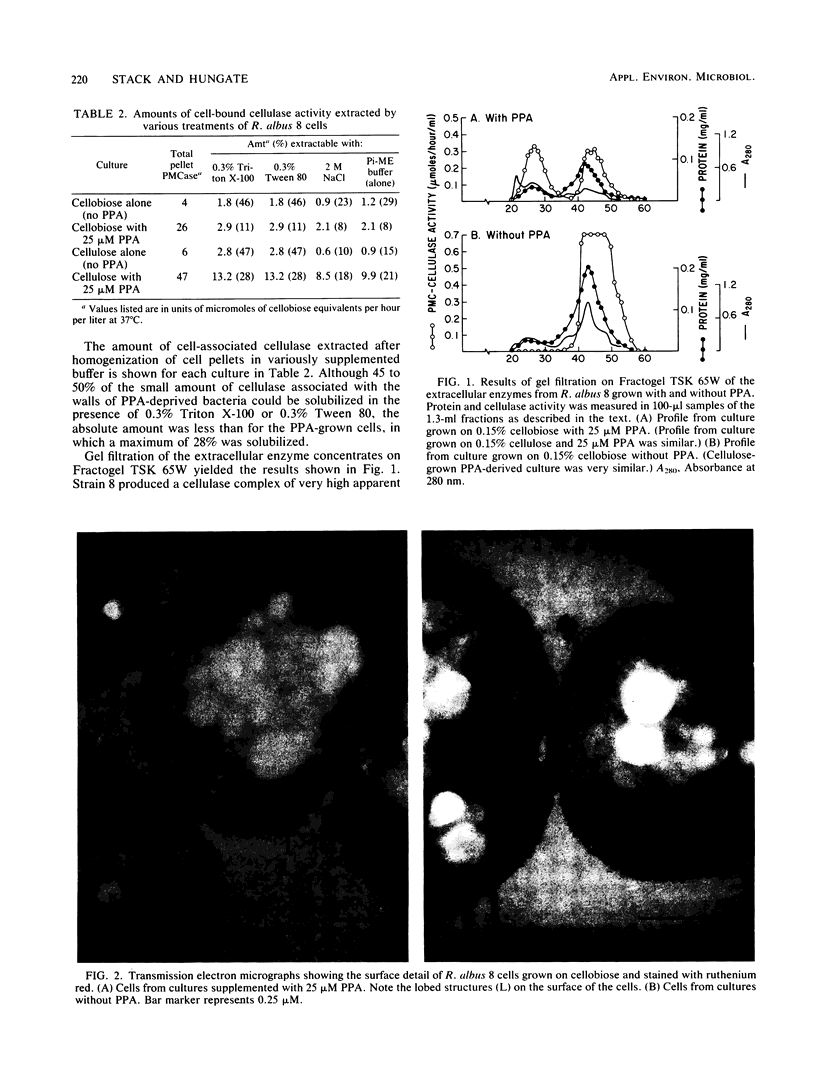
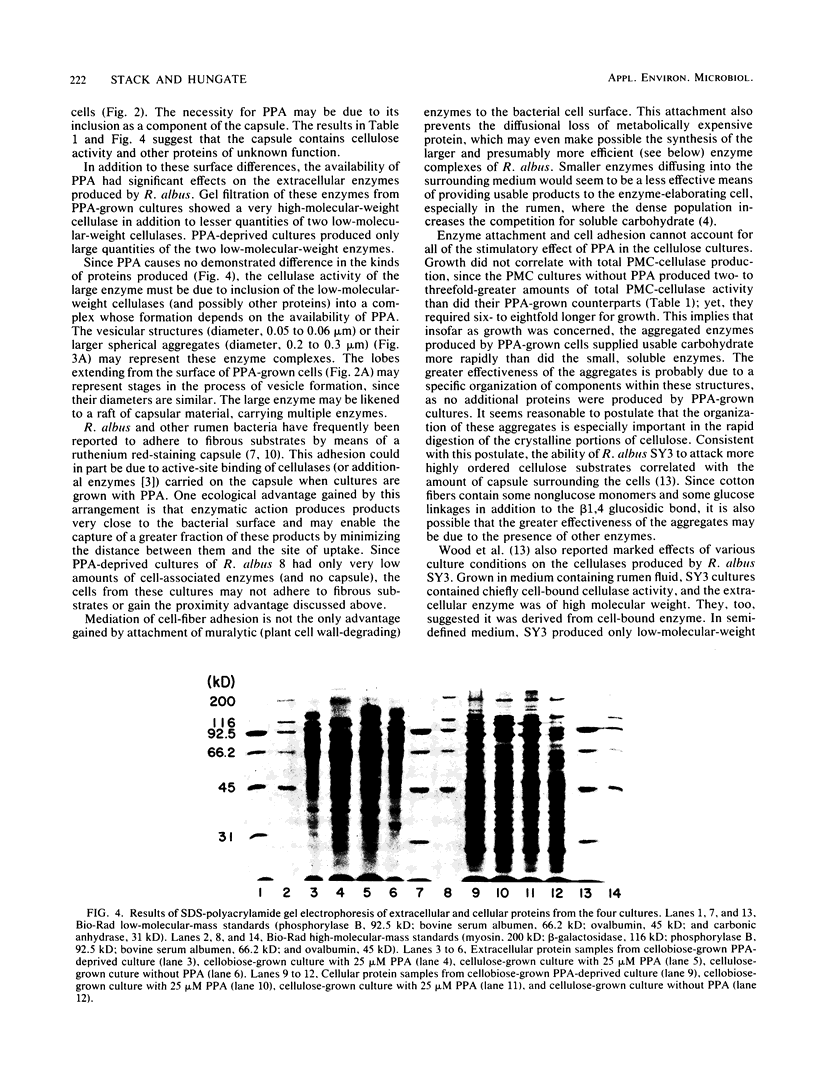
The morphology and cellulases of Ruminococcus albus 8 were markedly affected by the inclusion of 3-phenylpropanoic acid (PPA) in a defined growth medium. PPA-grown bacteria produced substantial quantities of cell-bound cellulase, as well as a very high-molecular-weight extracellular enzyme and lesser amounts of two low-molecular-weight enzymes. PPA-deprived bacteria produced greater total amounts of cellulase, but all of it exists in soluble, low-molecular-weight forms. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that the availability of PPA did not affect the kinds of proteins produced, but the distribution of two major proteins between cells and supernatant was PPA dependent. These two proteins (85 and 102 kilodaltons) were primarily associated with the cells of PPA-grown bacteria but were found chiefly in the supernatants of PPA-deprived cultures. Examination of thin sections of PPA-grown R. albus 8 by transmission electron microscopy showed a lobed ruthenium red-staining capsule surrounding the cell wall, as well as small vesicular structures (diameter, 0.05 to 0.06 μm) which appeared to aggregate into larger spherical units (diameter, 0.2 to 0.3 μm). In contrast, thin sections of PPA-deprived cells were devoid of vesicles and showed little or no capsule surrounding the cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Greve L. C., Labavitch J. M., Stack R. J., Hungate R. E. Muralytic Activities of Ruminococcus albus 8. Appl Environ Microbiol. 1984 May;47(5):1141–1145. doi: 10.1128/aem.47.5.1141-1145.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E., Stack R. J. Phenylpropanoic Acid: Growth Factor for Ruminococcus albus. Appl Environ Microbiol. 1982 Jul;44(1):79–83. doi: 10.1128/aem.44.1.79-83.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leatherwood J. M. Cellulose degradation by Ruminococcus. Fed Proc. 1973 Jul;32(7):1814–1818. [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T. W., Ward P. F., Dawson R. M. The formation and metabolism of phenyl-substituted fatty acids in the ruminant. Biochem J. 1964 Jan;90(1):12–24. doi: 10.1042/bj0900012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack R. J., Hungate R. E., Opsahl W. P. Phenylacetic acid stimulation of cellulose digestion by Ruminococcus albus 8. Appl Environ Microbiol. 1983 Sep;46(3):539–544. doi: 10.1128/aem.46.3.539-544.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M., Wilson C. A., Stewart C. S. Preparation of the cellulase from the cellulolytic anaerobic rumen bacterium Ruminococcus albus and its release from the bacterial cell wall. Biochem J. 1982 Jul 1;205(1):129–137. doi: 10.1042/bj2050129. [DOI] [PMC free article] [PubMed] [Google Scholar]