Abstract
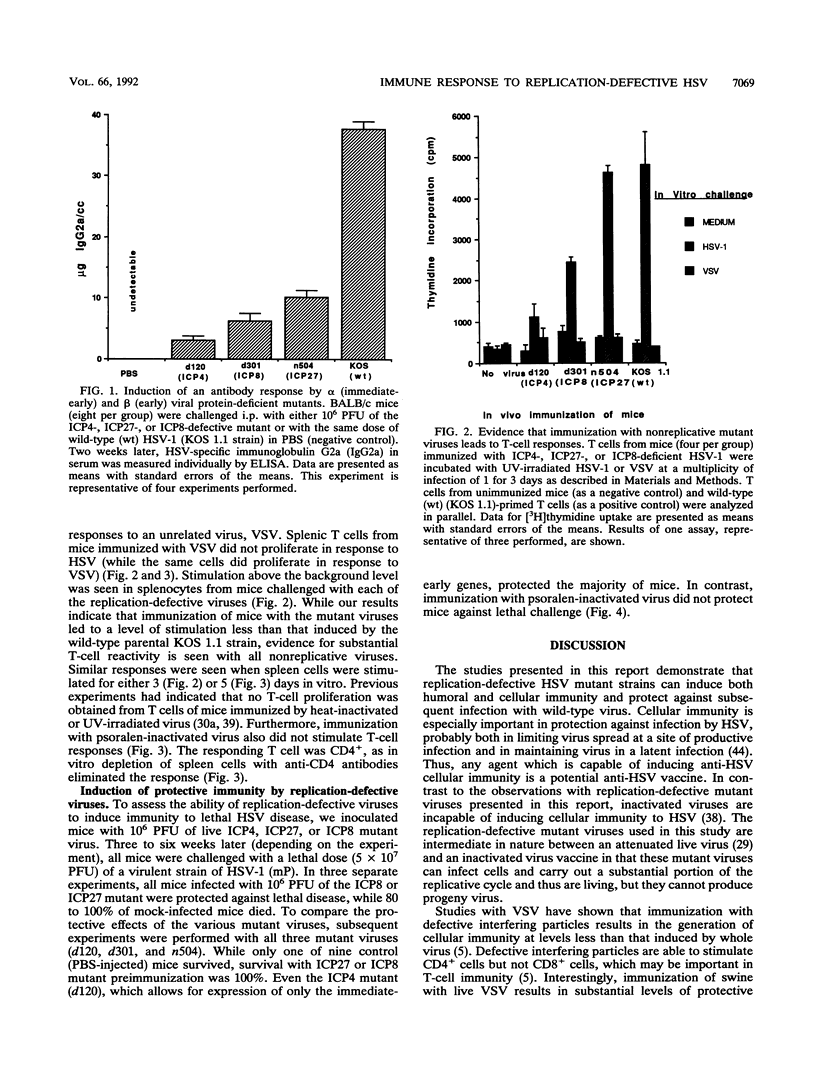
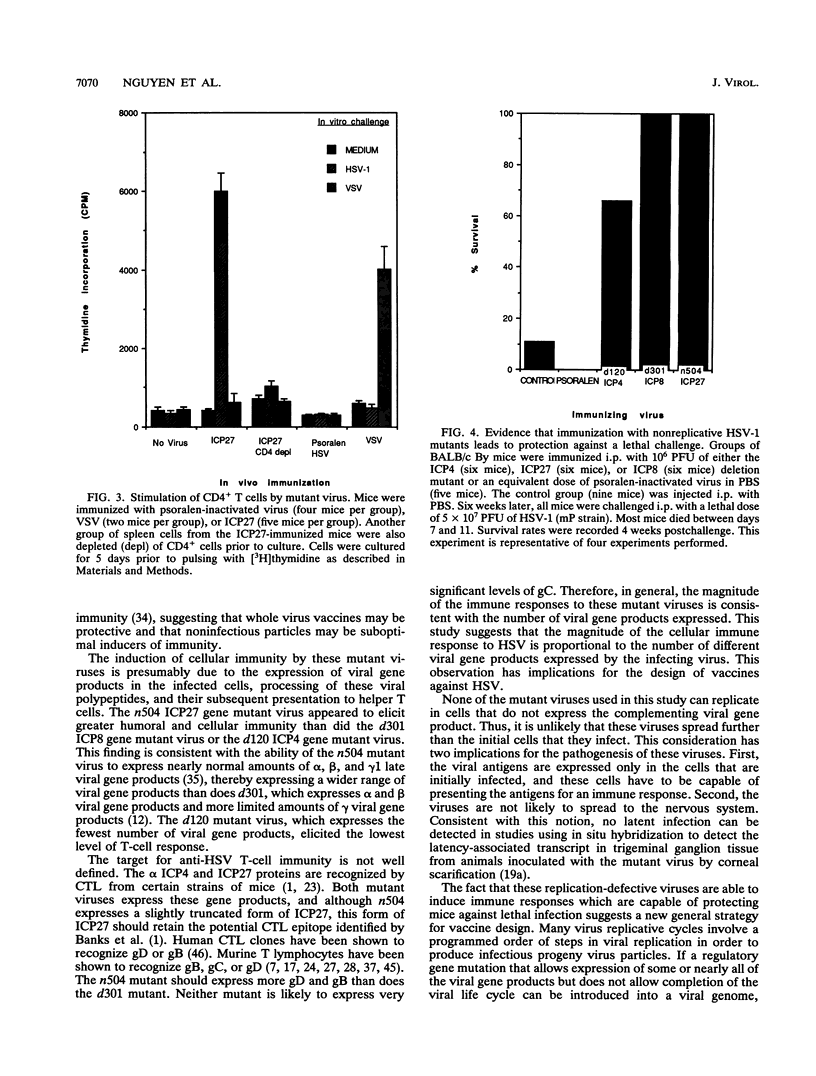
Live viruses and live virus vaccines induce cellular immunity more readily than do inactivated viruses or purified proteins, but the mechanism by which this process occurs is unknown. A trivial explanation would relate to the ability of live viruses to spread and infect more cells than can inactivated virus. We have used live but replication-defective mutants to investigate this question. Our studies indicate that the immune responses of mice to live virus differ greatly from the responses to inactivated virus even when the virus does not complete a replicative cycle. Further, these studies indicate that herpes simplex virus-specific T-cell responses can be generated by infection with replication-defective mutant viruses. These data indicate that the magnitude of the cellular immunity to herpes simplex virus may be proportional to the number or quantity of different viral gene products expressed by an immunizing virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks T. A., Allen E. M., Dasgupta S., Sandri-Goldin R., Rouse B. T. Herpes simplex virus type 1-specific cytotoxic T lymphocytes recognize immediate-early protein ICP27. J Virol. 1991 Jun;65(6):3185–3191. doi: 10.1128/jvi.65.6.3185-3191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman P. W., Gregory T., Crase D., Lasky L. A. Protection from genital herpes simplex virus type 2 infection by vaccination with cloned type 1 glycoprotein D. Science. 1985 Mar 22;227(4693):1490–1492. doi: 10.1126/science.2983428. [DOI] [PubMed] [Google Scholar]
- Blacklaws B. A., Nash A. A., Darby G. Specificity of the immune response of mice to herpes simplex virus glycoproteins B and D constitutively expressed on L cell lines. J Gen Virol. 1987 Apr;68(Pt 4):1103–1114. doi: 10.1099/0022-1317-68-4-1103. [DOI] [PubMed] [Google Scholar]
- Braun R. W., Teute H. K., Kirchner H., Munk K. Replication of herpes simplex virus in human T lymphocytes: characterization of the viral target cell. J Immunol. 1984 Feb;132(2):914–919. [PubMed] [Google Scholar]
- Browning M. J., Huneycutt B. S., Huang A. S., Reiss C. S. Replication-defective viruses modulate immune responses. J Immunol. 1991 Oct 15;147(8):2685–2691. [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Cantin E. M., Eberle R., Baldick J. L., Moss B., Willey D. E., Notkins A. L., Openshaw H. Expression of herpes simplex virus 1 glycoprotein B by a recombinant vaccinia virus and protection of mice against lethal herpes simplex virus 1 infection. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5908–5912. doi: 10.1073/pnas.84.16.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M., Sasaki O., Yamada A., Imanishi J. Enhancement of the protective effect of inactivated influenza virus vaccine by cytokines. Vaccine. 1992;10(4):238–242. doi: 10.1016/0264-410x(92)90158-g. [DOI] [PubMed] [Google Scholar]
- Chan W. L., Tizard M. L., Faulkner L. Proliferative T-cell response to glycoprotein B of the human herpes viruses: the influence of MHC and sequence of infection on the pattern of cross-reactivity. Immunology. 1989 Sep;68(1):96–101. [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., McCarthy A. M., Schaffer P. A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985 Nov;56(2):558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P., Howell D. M., Pettera L., Tehrani S., Lopez C. Immediate-early gene expression is sufficient for induction of natural killer cell-mediated lysis of herpes simplex virus type 1-infected fibroblasts. J Virol. 1991 Jun;65(6):3151–3160. doi: 10.1128/jvi.65.6.3151-3160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Knipe D. M. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J Virol. 1989 Dec;63(12):5258–5267. doi: 10.1128/jvi.63.12.5258-5267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvich E. B. The age-dependent risk of postvaccination complications in vaccinees with smallpox vaccine. Vaccine. 1992;10(2):96–97. doi: 10.1016/0264-410x(92)90023-d. [DOI] [PubMed] [Google Scholar]
- Highlander S. L., Cai W. H., Person S., Levine M., Glorioso J. C. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J Virol. 1988 Jun;62(6):1881–1888. doi: 10.1128/jvi.62.6.1881-1888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom R. C., Soman G., Finberg R. Trojan horse lymphocytes: a vesicular stomatitis virus-specific T-cell clone lyses target cells by carrying virus. J Virol. 1989 Oct;63(10):4157–4164. doi: 10.1128/jvi.63.10.4157-4164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Lancki D. W., Fitch F. W., Spear P. G. Herpes simplex virus glycoprotein D is recognized as antigen by CD4+ and CD8+ T lymphocytes from infected mice. Characterization of T cell clones. J Immunol. 1990 Jul 15;145(2):702–710. [PubMed] [Google Scholar]
- Kahlon J., Whitley R. J. Antibody response of the newborn after herpes simplex virus infection. J Infect Dis. 1988 Nov;158(5):925–933. doi: 10.1093/infdis/158.5.925. [DOI] [PubMed] [Google Scholar]
- Kohl S. Herpes simplex virus immunology: problems, progress, and promises. J Infect Dis. 1985 Sep;152(3):435–440. doi: 10.1093/infdis/152.3.435. [DOI] [PubMed] [Google Scholar]
- Kühn J. E., Dunkler G., Munk K., Braun R. W. Analysis of the IgM and IgG antibody response against herpes simplex virus type 1 (HSV-1) structural and nonstructural proteins. J Med Virol. 1987 Oct;23(2):135–150. doi: 10.1002/jmv.1890230206. [DOI] [PubMed] [Google Scholar]
- Lane J. M., Millar J. D. Risks of smallpox vaccination complications in the United States. Am J Epidemiol. 1971 Apr;93(4):238–240. doi: 10.1093/oxfordjournals.aje.a121252. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Courtney R. J., Fowler G., Rouse B. T. Herpes simplex virus type 1-specific cytotoxic T lymphocytes recognize virus nonstructural proteins. J Virol. 1988 Jul;62(7):2265–2273. doi: 10.1128/jvi.62.7.2265-2273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Moss B., Berman P. W., Laskey L. A., Rouse B. T. Mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: cytotoxic T cells. J Virol. 1987 Mar;61(3):726–734. doi: 10.1128/jvi.61.3.726-734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Zhu X. X., Silverstein S. J., Courtney R. J., Yao F., Jenkins F. J., Rouse B. T. Murine cytotoxic T lymphocytes specific for herpes simplex virus type 1 recognize the immediate early protein ICP4 but not ICP0. J Gen Virol. 1990 Oct;71(Pt 10):2391–2399. doi: 10.1099/0022-1317-71-10-2391. [DOI] [PubMed] [Google Scholar]
- McCarthy A. M., McMahan L., Schaffer P. A. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989 Jan;63(1):18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. R., Graham F. L., Hanke T., Johnson D. C. Protection of mice against lethal challenge with herpes simplex virus by vaccination with an adenovirus vector expressing HSV glycoprotein B. Virology. 1989 Mar;169(1):244–247. doi: 10.1016/0042-6822(89)90064-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin-Taylor E., Willey D. E., Cantin E. M., Eberle R., Moss B., Openshaw H. A recombinant vaccinia virus expressing herpes simplex virus type 1 glycoprotein B induces cytotoxic T lymphocytes in mice. J Gen Virol. 1988 Jul;69(Pt 7):1731–1734. doi: 10.1099/0022-1317-69-7-1731. [DOI] [PubMed] [Google Scholar]
- Meignier B., Longnecker R., Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis. 1988 Sep;158(3):602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R., Samsonoff C., Mercer S., Panicali D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1984 Jan;81(1):193–197. doi: 10.1073/pnas.81.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Knipe D. M. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol Cell Biol. 1985 May;5(5):957–963. doi: 10.1128/mcb.5.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelman D., Nichol S., Klieforth R., Van Der Maaten M., Whetstone C. Experimental vesicular stomatitis virus infection of swine: extent of infection and immunological response. Vet Immunol Immunopathol. 1989 Mar;20(4):345–361. doi: 10.1016/0165-2427(89)90080-9. [DOI] [PubMed] [Google Scholar]
- Rooney J. F., Wohlenberg C., Cremer K. J., Moss B., Notkins A. L. Immunization with a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: long-term protection and effect of revaccination. J Virol. 1988 May;62(5):1530–1534. doi: 10.1128/jvi.62.5.1530-1534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D. S., Rouse B. T. Cellular interactions in the cytotoxic T lymphocyte response to herpes simplex virus antigens: differential antigen activation requirements for the helper T lymphocyte and cytotoxic T lymphocyte precursors. J Immunol. 1983 Jul;131(1):479–484. [PubMed] [Google Scholar]
- Stanberry L. R., Bernstein D. I., Burke R. L., Pachl C., Myers M. G. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1987 May;155(5):914–920. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- Wachsman M., Aurelian L., Smith C. C., Perkus M. E., Paoletti E. Regulation of expression of herpes simplex virus (HSV) glycoprotein D in vaccinia recombinants affects their ability to protect from cutaneous HSV-2 disease. J Infect Dis. 1989 Apr;159(4):625–634. doi: 10.1093/infdis/159.4.625. [DOI] [PubMed] [Google Scholar]
- Webb S. R., Okamoto A., Ron Y., Sprent J. Restricted tissue distribution of Mlsa determinants. Stimulation of Mlsa-reactive T cells by B cells but not by dendritic cells or macrophages. J Exp Med. 1989 Jan 1;169(1):1–12. doi: 10.1084/jem.169.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Merigan T. C. Recombinant interleukin 2 as an adjuvant for vaccine-induced protection. Immunization of guinea pigs with herpes simplex virus subunit vaccines. J Immunol. 1988 Jan 1;140(1):294–299. [PubMed] [Google Scholar]
- Witmer L. A., Rosenthal K. L., Graham F. L., Friedman H. M., Yee A., Johnson D. C. Cytotoxic T lymphocytes specific for herpes simplex virus (HSV) studied using adenovirus vectors expressing HSV glycoproteins. J Gen Virol. 1990 Feb;71(Pt 2):387–396. doi: 10.1099/0022-1317-71-2-387. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Burke R. L., Pachl C., Berman P. W., Lasky L. A. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD. J Immunol. 1986 Jun 15;136(12):4669–4673. [PubMed] [Google Scholar]



