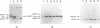Abstract
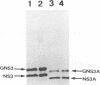
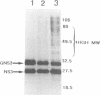
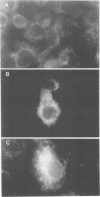
The genome of bluetongue virus, an orbivirus, consists of 10 double-stranded RNAs, each encoding at least one polypeptide. The smallest RNA segment (S10) encodes two minor nonstructural proteins, NS3 and NS3A, the structures and functions of which are not understood. We have expressed these two proteins in mammalian cells by using the T7 cytoplasmic transient expression system. Using a deletion mutant (lacking the first AUG initiation codon), we have demonstrated that the second initiation codon is used to initiate the synthesis of NS3A protein and that the two initiation codons are responsible for the synthesis not only of NS3 and NS3A but also of high-molecular-weight forms of both proteins. These higher-molecular-weight forms (GNS3 and GNS3A) are glycosylated. We have also demonstrated that the carbohydrate chains of GNS3 and GNS3A could be further modified by heterogeneous extension to polylactosaminoglycan forms. The glycosylated and nonglycosylated forms are found in similar intracellular locations in the Golgi complex. In the presence of cycloheximide, NS3 and NS3A immunofluorescence staining was pronounced in the Golgi complex, confirming that NS3 and NS3A are competent for transport to the Golgi apparatus after synthesis. We conclude that S10 gene products are integral membrane glycoproteins.
Full text
PDF



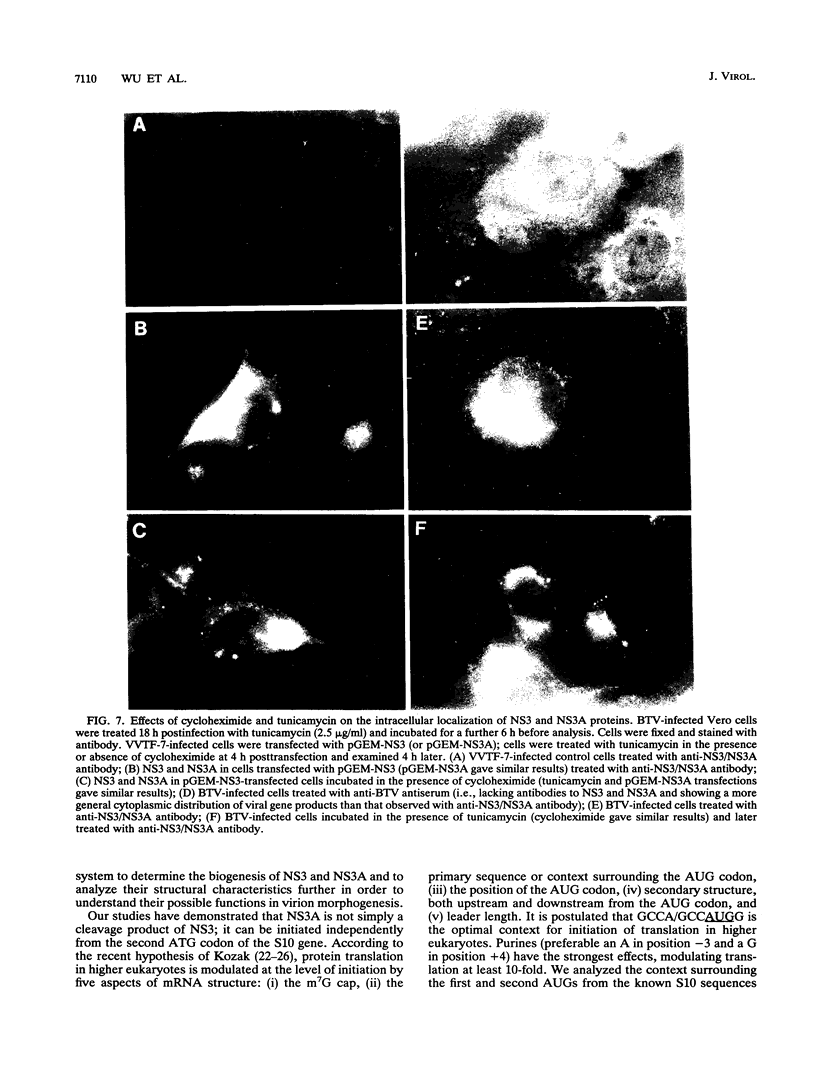
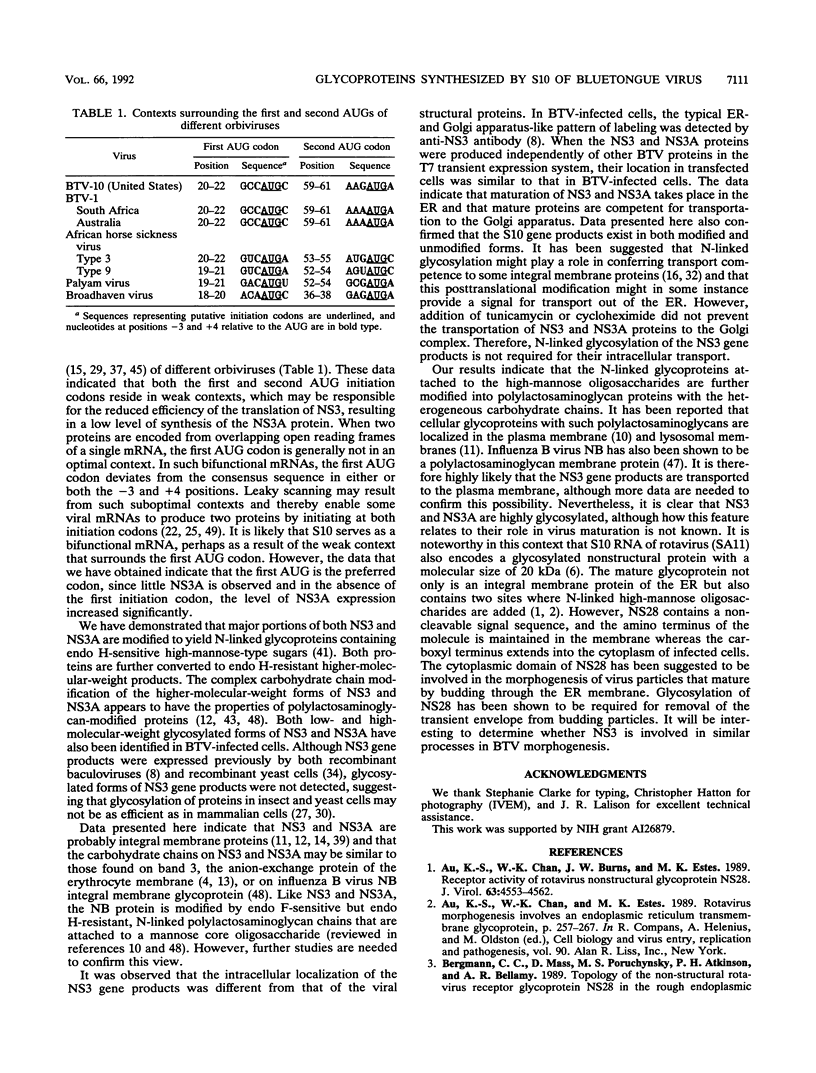

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au K. S., Chan W. K., Burns J. W., Estes M. K. Receptor activity of rotavirus nonstructural glycoprotein NS28. J Virol. 1989 Nov;63(11):4553–4562. doi: 10.1128/jvi.63.11.4553-4562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C. J., Tanner M. J., Kempf C. The human erythrocyte anion-transport protein. Partial amino acid sequence, conformation and a possible molecular mechanism for anion exchange. Biochem J. 1983 Sep 1;213(3):577–586. doi: 10.1042/bj2130577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton B. T., Hyatt A. D., Brookes S. M. The replication of bluetongue virus. Curr Top Microbiol Immunol. 1990;162:89–118. doi: 10.1007/978-3-642-75247-6_4. [DOI] [PubMed] [Google Scholar]
- Ericson B. L., Graham D. Y., Mason B. B., Hanssen H. H., Estes M. K. Two types of glycoprotein precursors are produced by the simian rotavirus SA11. Virology. 1983 Jun;127(2):320–332. doi: 10.1016/0042-6822(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French T. J., Inumaru S., Roy P. Expression of two related nonstructural proteins of bluetongue virus (BTV) type 10 in insect cells by a recombinant baculovirus: production of polyclonal ascitic fluid and characterization of the gene product in BTV-infected BHK cells. J Virol. 1989 Aug;63(8):3270–3278. doi: 10.1128/jvi.63.8.3270-3278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Bothner B., Ramsamooj P., Dell A., Tiller P. R., Varki A., Klock J. C. Structures of sialylated fucosyl polylactosaminoglycans isolated from chronic myelogenous leukemia cells. J Biol Chem. 1985 Oct 25;260(24):12957–12967. [PubMed] [Google Scholar]
- Fukuda M. Cell surface glycoconjugates as onco-differentiation markers in hematopoietic cells. Biochim Biophys Acta. 1985;780(2):119–150. doi: 10.1016/0304-419x(84)90002-7. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Dell A., Oates J. E., Fukuda M. N. Structure of branched lactosaminoglycan, the carbohydrate moiety of band 3 isolated from adult human erythrocytes. J Biol Chem. 1984 Jul 10;259(13):8260–8273. [PubMed] [Google Scholar]
- Fukuda M., Guan J. L., Rose J. K. A membrane-anchored form but not the secretory form of human chorionic gonadotropin-alpha chain acquires polylactosaminoglycan. J Biol Chem. 1988 Apr 15;263(11):5314–5318. [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991 Nov 15;266(32):21327–21330. [PubMed] [Google Scholar]
- Gould A. R. Nucleotide sequence of the Australian bluetongue virus serotype 1 RNA segment 10. J Gen Virol. 1988 Apr;69(Pt 4):945–949. doi: 10.1099/0022-1317-69-4-945. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Machamer C. E., Rose J. K. Glycosylation allows cell-surface transport of an anchored secretory protein. Cell. 1985 Sep;42(2):489–496. doi: 10.1016/0092-8674(85)90106-0. [DOI] [PubMed] [Google Scholar]
- Hewat E. A., Booth T. F., Loudon P. T., Roy P. Three-dimensional reconstruction of baculovirus expressed bluetongue virus core-like particles by cryo-electron microscopy. Virology. 1992 Jul;189(1):10–20. doi: 10.1016/0042-6822(92)90676-g. [DOI] [PubMed] [Google Scholar]
- Huismans H., Els H. J. Characterization of the tubules associated with the replication of three different orbiviruses. Virology. 1979 Jan 30;92(2):397–406. doi: 10.1016/0042-6822(79)90144-2. [DOI] [PubMed] [Google Scholar]
- Huismans H. Protein synthesis in bluetongue virus-infected cells. Virology. 1979 Jan 30;92(2):385–396. doi: 10.1016/0042-6822(79)90143-0. [DOI] [PubMed] [Google Scholar]
- Hyatt A. D., Gould A. R., Coupar B., Eaton B. T. Localization of the non-structural protein NS3 in bluetongue virus-infected cells. J Gen Virol. 1991 Sep;72(Pt 9):2263–2267. doi: 10.1099/0022-1317-72-9-2263. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Regulation of protein synthesis in virus-infected animal cells. Adv Virus Res. 1986;31:229–292. doi: 10.1016/S0065-3527(08)60265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukuruzinska M. A., Bergh M. L., Jackson B. J. Protein glycosylation in yeast. Annu Rev Biochem. 1987;56:915–944. doi: 10.1146/annurev.bi.56.070187.004411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. W., Roy P. Nucleotide sequence of a cDNA clone of RNA segment 10 of bluetongue virus (serotype 10). J Gen Virol. 1986 Dec;67(Pt 12):2833–2837. doi: 10.1099/0022-1317-67-12-2833. [DOI] [PubMed] [Google Scholar]
- Livi G. P., Ferrara A. A., Roskin R., Simon P. L., Young P. R. Secretion of N-glycosylated human recombinant interleukin-1 alpha in Saccharomyces cerevisiae. Gene. 1990 Apr 16;88(2):297–301. doi: 10.1016/0378-1119(90)90048-v. [DOI] [PubMed] [Google Scholar]
- Loudon P. T., Roy P. Assembly of five bluetongue virus proteins expressed by recombinant baculoviruses: inclusion of the largest protein VP1 in the core and virus-like proteins. Virology. 1991 Feb;180(2):798–802. doi: 10.1016/0042-6822(91)90094-r. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Florkiewicz R. Z., Rose J. K. A single N-linked oligosaccharide at either of the two normal sites is sufficient for transport of vesicular stomatitis virus G protein to the cell surface. Mol Cell Biol. 1985 Nov;5(11):3074–3083. doi: 10.1128/mcb.5.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn J. C., Gould A. R., Eaton B. T. High level expression of the major core protein VP7 and the non-structural protein NS3 of bluetongue virus in yeast: use of expressed VP7 as a diagnostic, group-reactive antigen in a blocking ELISA. Virus Res. 1991 Mar;18(2-3):165–178. doi: 10.1016/0168-1702(91)90016-o. [DOI] [PubMed] [Google Scholar]
- Mertens P. P., Brown F., Sangar D. V. Assignment of the genome segments of bluetongue virus type 1 to the proteins which they encode. Virology. 1984 May;135(1):207–217. doi: 10.1016/0042-6822(84)90131-4. [DOI] [PubMed] [Google Scholar]
- Meyer J. C., Bergmann C. C., Bellamy A. R. Interaction of rotavirus cores with the nonstructural glycoprotein NS28. Virology. 1989 Jul;171(1):98–107. doi: 10.1016/0042-6822(89)90515-1. [DOI] [PubMed] [Google Scholar]
- Moss S. R., Jones L. D., Nuttall P. A. Comparison of the nonstructural protein, NS3, of tick-borne and insect-borne orbiviruses. Virology. 1992 Apr;187(2):841–844. doi: 10.1016/0042-6822(92)90491-7. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Yamaguchi S., Roy P. Three-dimensional structure of single-shelled bluetongue virus. J Virol. 1992 Apr;66(4):2135–2142. doi: 10.1128/jvi.66.4.2135-2142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Roy P., Marshall J. J., French T. J. Structure of the bluetongue virus genome and its encoded proteins. Curr Top Microbiol Immunol. 1990;162:43–87. doi: 10.1007/978-3-642-75247-6_3. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Thomas C. P., Booth T. F., Roy P. Synthesis of bluetongue virus-encoded phosphoprotein and formation of inclusion bodies by recombinant baculovirus in insect cells: it binds the single-stranded RNA species. J Gen Virol. 1990 Sep;71(Pt 9):2073–2083. doi: 10.1099/0022-1317-71-9-2073. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Optimizing hydrolysis of N-linked high-mannose oligosaccharides by endo-beta-N-acetylglucosaminidase H. Anal Biochem. 1984 Sep;141(2):515–522. doi: 10.1016/0003-2697(84)90080-0. [DOI] [PubMed] [Google Scholar]
- Urakawa T., Roy P. Bluetongue virus tubules made in insect cells by recombinant baculoviruses: expression of the NS1 gene of bluetongue virus serotype 10. J Virol. 1988 Nov;62(11):3919–3927. doi: 10.1128/jvi.62.11.3919-3927.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd D. W., Els H. J., De Villiers E. M., Huismans H. Structure of the bluetongue virus capsid. J Virol. 1972 Oct;10(4):783–794. doi: 10.1128/jvi.10.4.783-794.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. A., Lamb R. A. Determination of the orientation of an integral membrane protein and sites of glycosylation by oligonucleotide-directed mutagenesis: influenza B virus NB glycoprotein lacks a cleavable signal sequence and has an extracellular NH2-terminal region. Mol Cell Biol. 1986 Dec;6(12):4317–4328. doi: 10.1128/mcb.6.12.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. A., Lamb R. A. Effect of mutations and deletions in a bicistronic mRNA on the synthesis of influenza B virus NB and NA glycoproteins. J Virol. 1989 Jan;63(1):28–35. doi: 10.1128/jvi.63.1.28-35.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. A., Lamb R. A. Polylactosaminoglycan modification of a small integral membrane glycoprotein, influenza B virus NB. Mol Cell Biol. 1988 Mar;8(3):1186–1196. doi: 10.1128/mcb.8.3.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staden V., Huismans H. A comparison of the genes which encode non-structural protein NS3 of different orbiviruses. J Gen Virol. 1991 May;72(Pt 5):1073–1079. doi: 10.1099/0022-1317-72-5-1073. [DOI] [PubMed] [Google Scholar]