Abstract
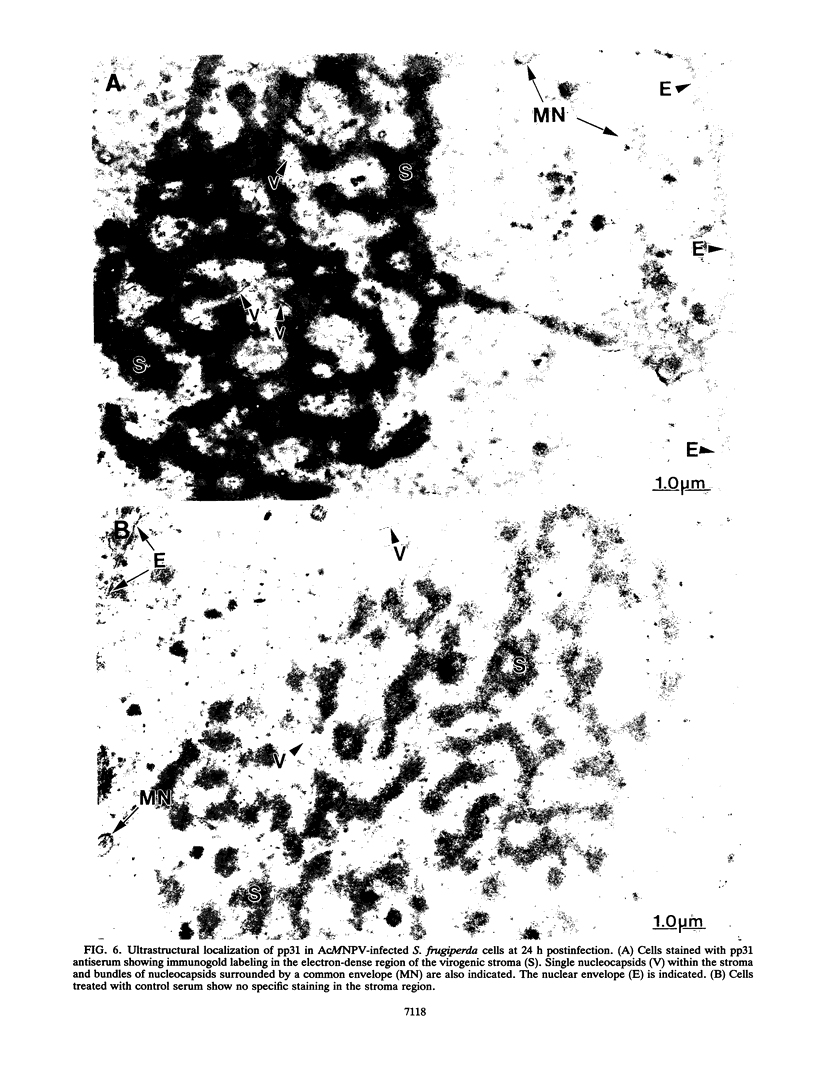
The PstI K fragment of Autographa californica nuclear polyhedrosis virus (AcMNPV) encodes a protein with a molecular weight of 31,000. To define the role of this protein (pp31) in virus infection further, it was overexpressed in bacteria and used to produce polyclonal antiserum. Radioimmunoprecipitation analysis indicated that pp31 was synthesized during both the early and late phases of virus infection, consistent with previous analyses indicating that the gene was regulated by tandem early and late promoters. Metabolic labeling of cells with carrier-free phosphate indicated that pp31 was phosphorylated. Biochemical fractionation experiments showed that pp31 was localized in the nucleus and that it was more stably associated with the nucleus at later times of infection. Immunoblot analysis of subnuclear fractions indicated that pp31 was associated predominantly with the chromatin and nuclear matrix fractions. Immunofluorescence experiments confirmed that the pp31 protein was localized in the nucleus. Nuclear staining was relatively uniform early but was more centrally nuclear later in infection. Immunoelectron microscopy indicated that the pp31 protein was a component of virogenic stroma. Southwestern (DNA-protein) blot analysis demonstrated that pp31 is a DNA-binding protein. These findings suggest a possible role for pp31 in the virus life cycle.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berezney R. The nuclear matrix: a heuristic model for investigating genomic organization and function in the cell nucleus. J Cell Biochem. 1991 Oct;47(2):109–123. doi: 10.1002/jcb.240470204. [DOI] [PubMed] [Google Scholar]
- Blissard G. W., Quant-Russell R. L., Rohrmann G. F., Beaudreau G. S. Nucleotide sequence, transcriptional mapping, and temporal expression of the gene encoding p39, a major structural protein of the multicapsid nuclear polyhedrosis virus of Orgyia pseudotsugata. Virology. 1989 Feb;168(2):354–362. doi: 10.1016/0042-6822(89)90276-6. [DOI] [PubMed] [Google Scholar]
- Carson D. D., Guarino L. A., Summers M. D. Functional mapping of an AcNPV immediately early gene which augments expression of the IE-1 trans-activated 39K gene. Virology. 1988 Feb;162(2):444–451. doi: 10.1016/0042-6822(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Smith M. W. Nucleotide sequence and characterization of the 39K gene region of Autographa californica nuclear polyhedrosis virus. Virology. 1990 Nov;179(1):1–8. doi: 10.1016/0042-6822(90)90266-t. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Smith M. Regulation of delayed-early gene transcription by dual TATA boxes. J Virol. 1992 Jun;66(6):3733–3739. doi: 10.1128/jvi.66.6.3733-3739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Summers M. D. Functional mapping of a trans-activating gene required for expression of a baculovirus delayed-early gene. J Virol. 1986 Feb;57(2):563–571. doi: 10.1128/jvi.57.2.563-571.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hoopes R. R., Jr, Rohrmann G. F. In vitro transcription of baculovirus immediate early genes: accurate mRNA initiation by nuclear extracts from both insect and human cells. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4513–4517. doi: 10.1073/pnas.88.10.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Imbalzano A. N., Shepard A. A., DeLuca N. A. Functional relevance of specific interactions between herpes simplex virus type 1 ICP4 and sequences from the promoter-regulatory domain of the viral thymidine kinase gene. J Virol. 1990 Jun;64(6):2620–2631. doi: 10.1128/jvi.64.6.2620-2631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis D. L., Bohlmeyer D. A., Garcia A., Jr Enhancement of polyhedrin nuclear localization during baculovirus infection. J Virol. 1992 Dec;66(12):6903–6911. doi: 10.1128/jvi.66.12.6903-6911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis D. L., Bohlmeyer D. A., Garcia A., Jr Requirements for nuclear localization and supramolecular assembly of a baculovirus polyhedrin protein. Virology. 1991 Dec;185(2):795–810. doi: 10.1016/0042-6822(91)90551-l. [DOI] [PubMed] [Google Scholar]
- Jarvis D. L., Summers M. D. Glycosylation and secretion of human tissue plasminogen activator in recombinant baculovirus-infected insect cells. Mol Cell Biol. 1989 Jan;9(1):214–223. doi: 10.1128/mcb.9.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kovacs G. R., Guarino L. A., Summers M. D. Novel regulatory properties of the IE1 and IE0 transactivators encoded by the baculovirus Autographa californica multicapsid nuclear polyhedrosis virus. J Virol. 1991 Oct;65(10):5281–5288. doi: 10.1128/jvi.65.10.5281-5288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Preparation of nuclear matrices from cultured cells: subfractionation of nuclei in situ. J Cell Biol. 1984 May;98(5):1886–1894. doi: 10.1083/jcb.98.5.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Theilmann D. A., Stewart S. Identification and characterization of the IE-1 gene of Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus. Virology. 1991 Feb;180(2):492–508. doi: 10.1016/0042-6822(91)90063-h. [DOI] [PubMed] [Google Scholar]
- Verheijen R., van Venrooij W., Ramaekers F. The nuclear matrix: structure and composition. J Cell Sci. 1988 May;90(Pt 1):11–36. doi: 10.1242/jcs.90.1.11. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Price K. H. Association of Autographa californica nuclear polyhedrosis virus (AcMNPV) with the nuclear matrix. Virology. 1988 Nov;167(1):233–241. doi: 10.1016/0042-6822(88)90073-6. [DOI] [PubMed] [Google Scholar]