Abstract
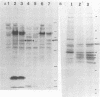
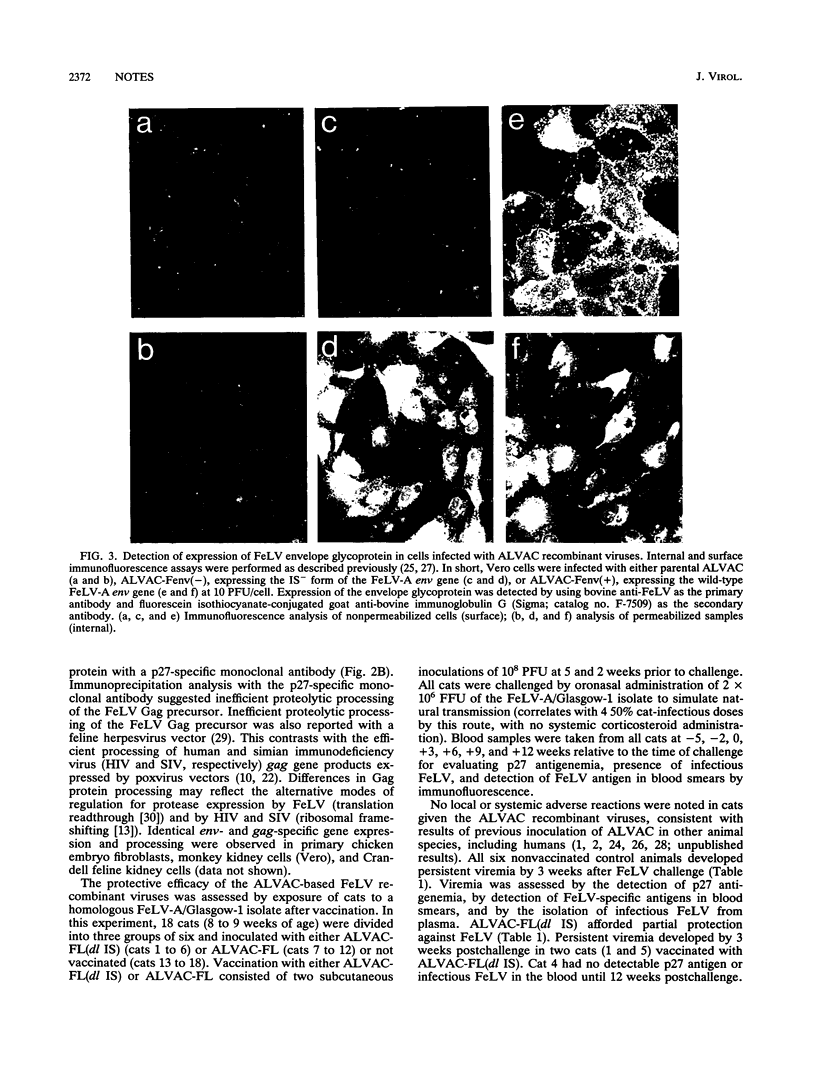
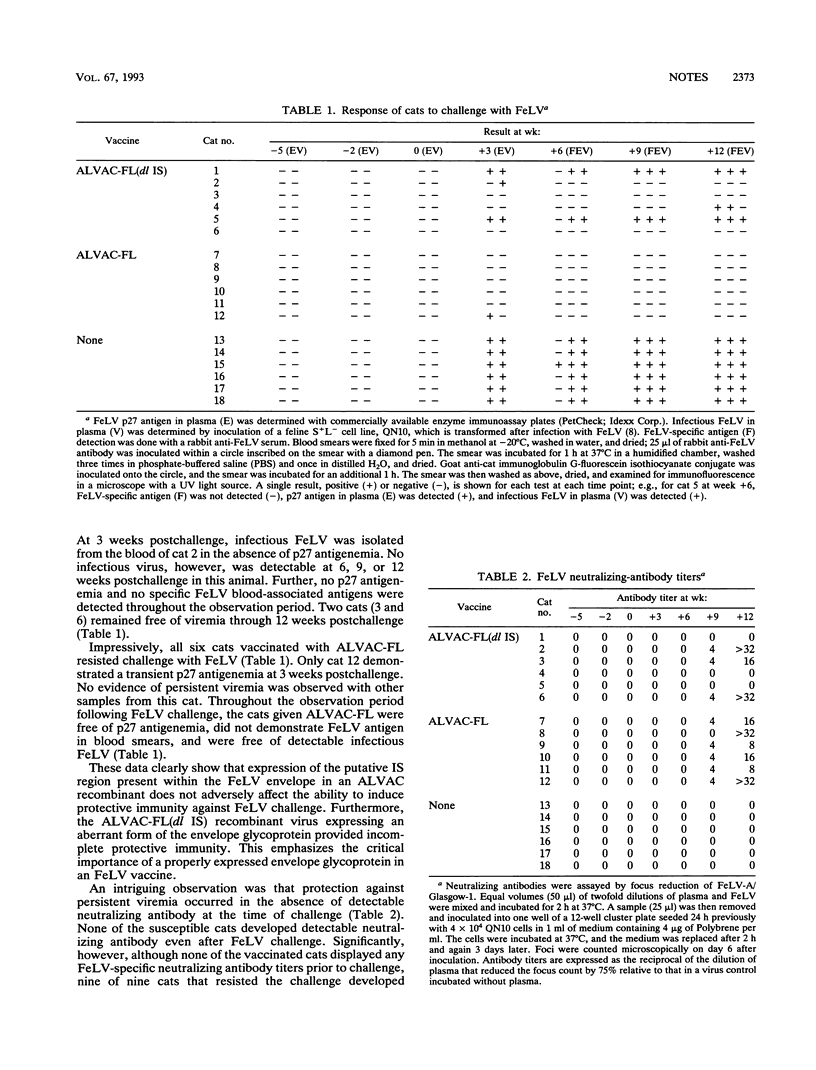
Two ALVAC (canarypox virus)-based recombinant viruses expressing the feline leukemia virus (FeLV) subgroup A env and gag genes were assessed for their protective efficacy in cats. Both recombinant viruses contained the entire gag gene. ALVAC-FL also expressed the entire envelope glycoprotein, while ALVAC-FL(dl IS) expressed an env-specific gene product deleted of the putative immunosuppressive region. Although only 50% of the cats vaccinated with ALVAC-FL(dl IS) were protected against persistent viremia after oronasal exposure to a homologous FeLV isolate, all cats administered ALVAC-FL resisted the challenge exposure. Significantly, protection was afforded in the absence of detectable FeLV-neutralizing antibodies. These results represent the first effective vaccination of cats against FeLV with a poxvirus-based recombinant vector and have implications that are relevant not only to FeLV vaccine development but also to developing vaccines against other retroviruses, including human immunodeficiency virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxby D., Paoletti E. Potential use of non-replicating vectors as recombinant vaccines. Vaccine. 1992;10(1):8–9. doi: 10.1016/0264-410x(92)90411-c. [DOI] [PubMed] [Google Scholar]
- Cadoz M., Strady A., Meignier B., Taylor J., Tartaglia J., Paoletti E., Plotkin S. Immunisation with canarypox virus expressing rabies glycoprotein. Lancet. 1992 Jun 13;339(8807):1429–1432. doi: 10.1016/0140-6736(92)92027-d. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985 Oct 25;230(4724):453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- Clark N., Kushner N. N., Barrett C. B., Kensil C. R., Salsbury D., Cotter S. Efficacy and safety field trials of a recombinant DNA vaccine against feline leukemia virus infection. J Am Vet Med Assoc. 1991 Nov 15;199(10):1433–1443. [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Morrison R. P., Wehrly K., Nishio J., Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986 Nov 7;234(4777):728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. H., Pedersen N. C., Nunberg J. H. Feline leukemia virus envelope protein expression encoded by a recombinant vaccinia virus: apparent lack of immunogenicity in vaccinated animals. Virus Res. 1987 Feb;7(1):49–67. doi: 10.1016/0168-1702(87)90057-8. [DOI] [PubMed] [Google Scholar]
- Haffar O., Garrigues J., Travis B., Moran P., Zarling J., Hu S. L. Human immunodeficiency virus-like, nonreplicating, gag-env particles assemble in a recombinant vaccinia virus expression system. J Virol. 1990 Jun;64(6):2653–2659. doi: 10.1128/jvi.64.6.2653-2659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover E. A., Perigo N. A., Quackenbush S. L., Mathiason-DuBard C. K., Overbaugh J. M., Kloetzer W. S., Elder J. H., Mullins J. I. Protection against feline leukemia virus infection by use of an inactivated virus vaccine. J Am Vet Med Assoc. 1991 Nov 15;199(10):1392–1401. [PubMed] [Google Scholar]
- Issel C. J., Horohov D. W., Lea D. F., Adams W. V., Jr, Hagius S. D., McManus J. M., Allison A. C., Montelaro R. C. Efficacy of inactivated whole-virus and subunit vaccines in preventing infection and disease caused by equine infectious anemia virus. J Virol. 1992 Jun;66(6):3398–3408. doi: 10.1128/jvi.66.6.3398-3408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Mandecki W. Oligonucleotide-directed double-strand break repair in plasmids of Escherichia coli: a method for site-specific mutagenesis. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7177–7181. doi: 10.1073/pnas.83.19.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M., Nishio J., Chesebro B. Protection against Friend retrovirus-induced leukemia by recombinant vaccinia viruses expressing the gag gene. J Virol. 1992 Jul;66(7):4497–4507. doi: 10.1128/jvi.66.7.4497-4507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. C., Johnson L., Birch D., Theilen G. H. Possible immunoenhancement of persistent viremia by feline leukemia virus envelope glycoprotein vaccines in challenge-exposure situations where whole inactivated virus vaccines were protective. Vet Immunol Immunopathol. 1986 Feb;11(2):123–148. doi: 10.1016/0165-2427(86)90093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkus M. E., Limbach K., Paoletti E. Cloning and expression of foreign genes in vaccinia virus, using a host range selection system. J Virol. 1989 Sep;63(9):3829–3836. doi: 10.1128/jvi.63.9.3829-3836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini A., Perkus M. E., Paoletti E. Vaccinia virus as an expression vector. Methods Enzymol. 1987;153:545–563. doi: 10.1016/0076-6879(87)53077-4. [DOI] [PubMed] [Google Scholar]
- Riviere M., Tartaglia J., Perkus M. E., Norton E. K., Bongermino C. M., Lacoste F., Duret C., Desmettre P., Paoletti E. Protection of mice and swine from pseudorabies virus conferred by vaccinia virus-based recombinants. J Virol. 1992 Jun;66(6):3424–3434. doi: 10.1128/jvi.66.6.3424-3434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. A., Warnock M., Wheeler A., Wilkie N., Mullins J. I., Onions D. E., Neil J. C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. J Virol. 1986 Jun;58(3):825–834. doi: 10.1128/jvi.58.3.825-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia J., Perkus M. E., Taylor J., Norton E. K., Audonnet J. C., Cox W. I., Davis S. W., van der Hoeven J., Meignier B., Riviere M. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992 May;188(1):217–232. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- Taylor J., Edbauer C., Rey-Senelonge A., Bouquet J. F., Norton E., Goebel S., Desmettre P., Paoletti E. Newcastle disease virus fusion protein expressed in a fowlpox virus recombinant confers protection in chickens. J Virol. 1990 Apr;64(4):1441–1450. doi: 10.1128/jvi.64.4.1441-1450.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J., Trimarchi C., Weinberg R., Languet B., Guillemin F., Desmettre P., Paoletti E. Efficacy studies on a canarypox-rabies recombinant virus. Vaccine. 1991 Mar;9(3):190–193. doi: 10.1016/0264-410x(91)90152-v. [DOI] [PubMed] [Google Scholar]
- Taylor J., Weinberg R., Kawaoka Y., Webster R. G., Paoletti E. Protective immunity against avian influenza induced by a fowlpox virus recombinant. Vaccine. 1988 Dec;6(6):504–508. doi: 10.1016/0264-410x(88)90101-6. [DOI] [PubMed] [Google Scholar]
- Taylor J., Weinberg R., Tartaglia J., Richardson C., Alkhatib G., Briedis D., Appel M., Norton E., Paoletti E. Nonreplicating viral vectors as potential vaccines: recombinant canarypox virus expressing measles virus fusion (F) and hemagglutinin (HA) glycoproteins. Virology. 1992 Mar;187(1):321–328. doi: 10.1016/0042-6822(92)90321-f. [DOI] [PubMed] [Google Scholar]
- Wardley R. C., Berlinski P. J., Thomsen D. R., Meyer A. L., Post L. E. The use of feline herpesvirus and baculovirus as vaccine vectors for the gag and env genes of feline leukaemia virus. J Gen Virol. 1992 Jul;73(Pt 7):1811–1818. doi: 10.1099/0022-1317-73-7-1811. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Translational readthrough of an amber termination codon during synthesis of feline leukemia virus protease. J Virol. 1985 Sep;55(3):870–873. doi: 10.1128/jvi.55.3.870-873.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]