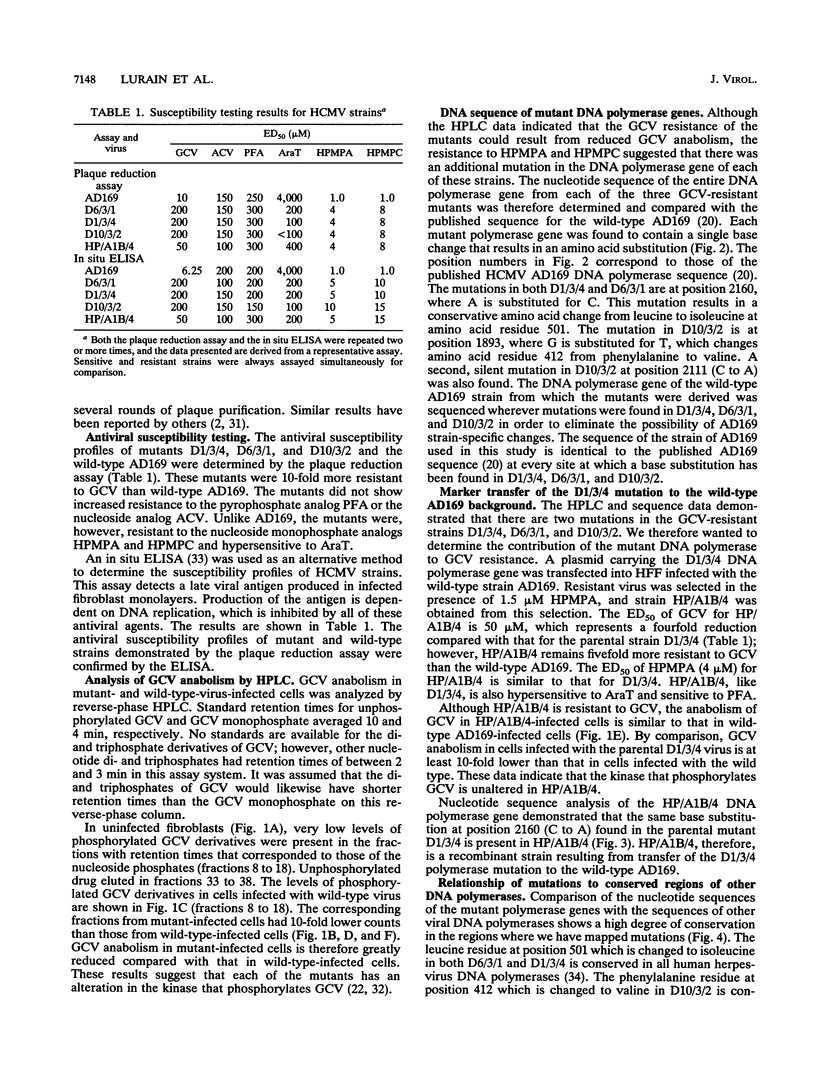
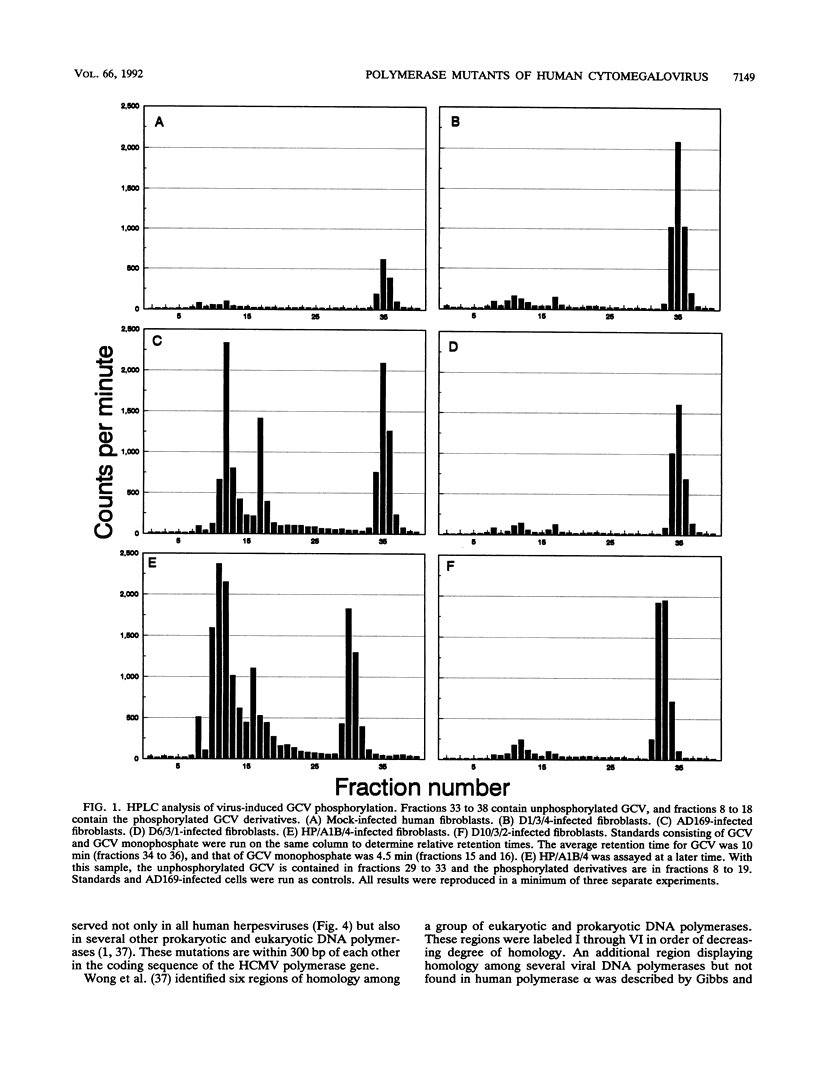
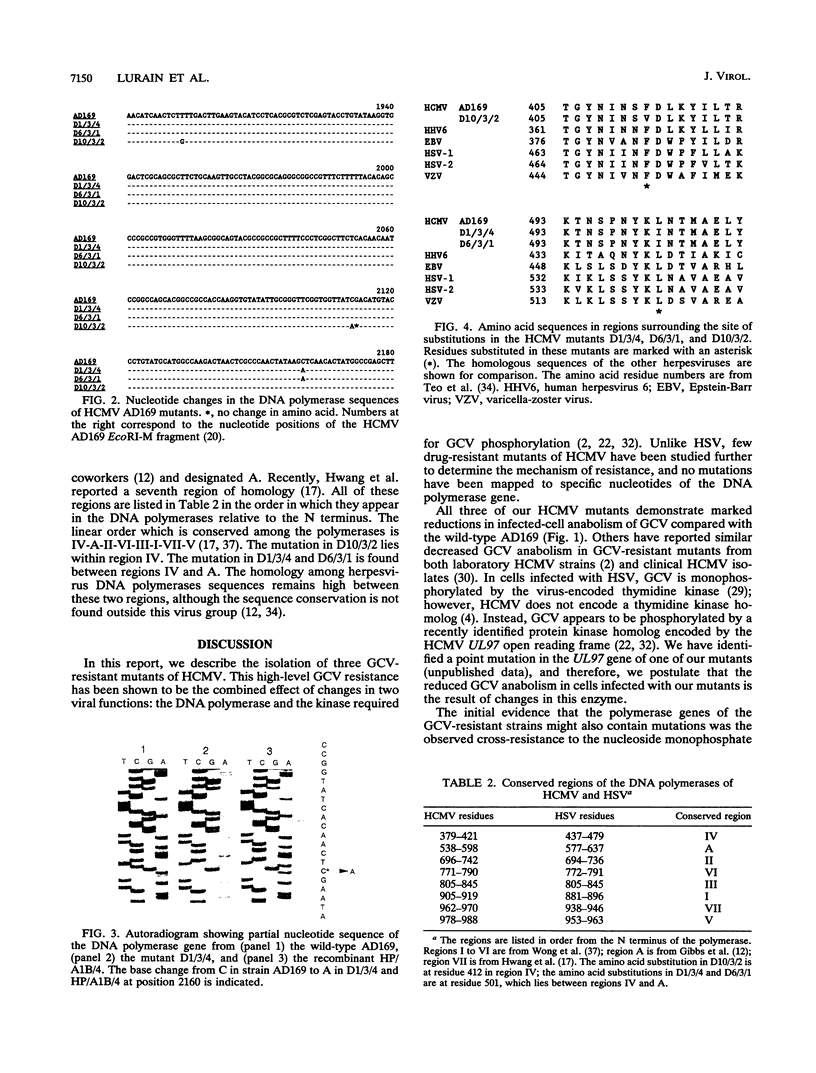
Abstract
Three independently isolated mutants of human cytomegalovirus strain AD169 were found to be resistant to ganciclovir at a 50% effective dose of 200 microM. Phosphorylation of ganciclovir was reduced 10-fold in mutant-infected cells compared with AD169-infected cells. All three mutants were also determined to be resistant to the nucleotide analogs (S)-1-[(3-hydroxy-2- phosphonylmethoxy)propyl]adenine (HPMPA) and (S)-1-[(3-hydroxy-2-phosphonylmethoxy)propyl]cytosine (HPMPC) and hypersensitive to thymine-1-D-arabinofuranoside (AraT). Single base changes resulting in amino acid substitutions were demonstrated in the nucleotide sequence of the DNA polymerase gene of each mutant. The polymerase mutation contained in one of the mutants was transferred to the wild-type AD169 background. Ganciclovir phosphorylation in cells infected with the recombinant virus produced by this transfer was found to be equivalent to that of AD169-infected cells. The ganciclovir resistance of the recombinant was reduced fourfold compared with that of the parental mutant; however, the recombinant remained resistant to HPMPA and HPMPC and hypersensitive to AraT. The ganciclovir resistance of the mutants therefore appears to result from mutations in two genes: (i) a kinase which phosphorylates ganciclovir and (ii) the viral DNA polymerase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernad A., Blanco L., Lázaro J. M., Martín G., Salas M. A conserved 3'----5' exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell. 1989 Oct 6;59(1):219–228. doi: 10.1016/0092-8674(89)90883-0. [DOI] [PubMed] [Google Scholar]
- Biron K. K., Fyfe J. A., Stanat S. C., Leslie L. K., Sorrell J. B., Lambe C. U., Coen D. M. A human cytomegalovirus mutant resistant to the nucleoside analog 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine (BW B759U) induces reduced levels of BW B759U triphosphate. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8769–8773. doi: 10.1073/pnas.83.22.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Bernad A., Blasco M. A., Salas M. A general structure for DNA-dependent DNA polymerases. Gene. 1991 Apr;100:27–38. doi: 10.1016/0378-1119(91)90346-d. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Fleming H. E., Jr, Leslie L. K., Retondo M. J. Sensitivity of arabinosyladenine-resistant mutants of herpes simplex virus to other antiviral drugs and mapping of drug hypersensitivity mutations to the DNA polymerase locus. J Virol. 1985 Feb;53(2):477–488. doi: 10.1128/jvi.53.2.477-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C. S., Kowalsky P. N., Oliver S. A., Schnipper L. E., Field A. K. Resistance of herpes simplex virus to 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]guanine: physical mapping of drug synergism within the viral DNA polymerase locus. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1556–1560. doi: 10.1073/pnas.81.5.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aquila R. T., Summers W. C. Isolation and characterization of phosphonoacetic acid-resistant mutants of human cytomegalovirus. J Virol. 1987 Apr;61(4):1291–1295. doi: 10.1128/jvi.61.4.1291-1295.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew W. L. Cytomegalovirus infection in patients with AIDS. J Infect Dis. 1988 Aug;158(2):449–456. doi: 10.1093/infdis/158.2.449. [DOI] [PubMed] [Google Scholar]
- Erice A., Chou S., Biron K. K., Stanat S. C., Balfour H. H., Jr, Jordan M. C. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N Engl J Med. 1989 Feb 2;320(5):289–293. doi: 10.1056/NEJM198902023200505. [DOI] [PubMed] [Google Scholar]
- Forbes B. A. Acquisition of cytomegalovirus infection: an update. Clin Microbiol Rev. 1989 Apr;2(2):204–216. doi: 10.1128/cmr.2.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. S., Chiou H. C., Bastow K. F., Cheng Y. C., Coen D. M. Identification of amino acids in herpes simplex virus DNA polymerase involved in substrate and drug recognition. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6672–6676. doi: 10.1073/pnas.85.18.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. S., Chiou H. C., Hall J. D., Mount D. W., Retondo M. J., Weller S. K., Coen D. M. Sequence and mapping analyses of the herpes simplex virus DNA polymerase gene predict a C-terminal substrate binding domain. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7969–7973. doi: 10.1073/pnas.82.23.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. S., Weisshart K., Digard P., deBruynKops A., Knipe D. M., Coen D. M. Polymerization activity of an alpha-like DNA polymerase requires a conserved 3'-5' exonuclease active site. Mol Cell Biol. 1991 Sep;11(9):4786–4795. doi: 10.1128/mcb.11.9.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D., Larder B. A., Inglis M. M. Evidence that the 'active centre' of the herpes simplex virus thymidine kinase involves an interaction between three distinct regions of the polypeptide. J Gen Virol. 1986 Apr;67(Pt 4):753–758. doi: 10.1099/0022-1317-67-4-753. [DOI] [PubMed] [Google Scholar]
- Haffey M. L., Novotny J., Bruccoleri R. E., Carroll R. D., Stevens J. T., Matthews J. T. Structure-function studies of the herpes simplex virus type 1 DNA polymerase. J Virol. 1990 Oct;64(10):5008–5018. doi: 10.1128/jvi.64.10.5008-5018.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Purifoy D. J., Young D., Gopal R., Cammack N., O'Hare P. Single mutations at many sites within the DNA polymerase locus of herpes simplex viruses can confer hypersensitivity to aphidicolin and resistance to phosphonoacetic acid. J Gen Virol. 1984 Jan;65(Pt 1):1–17. doi: 10.1099/0022-1317-65-1-1. [DOI] [PubMed] [Google Scholar]
- Hwang C. B., Ruffner K. L., Coen D. M. A point mutation within a distinct conserved region of the herpes simplex virus DNA polymerase gene confers drug resistance. J Virol. 1992 Mar;66(3):1774–1776. doi: 10.1128/jvi.66.3.1774-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. A., Drew W. L., Feinberg J., O'Donnell J. J., Whitmore P. V., Miner R. D., Parenti D. Foscarnet therapy for ganciclovir-resistant cytomegalovirus retinitis in patients with AIDS. J Infect Dis. 1991 Jun;163(6):1348–1351. doi: 10.1093/infdis/163.6.1348. [DOI] [PubMed] [Google Scholar]
- Knopf C. W., Weisshart K. Comparison of exonucleolytic activities of herpes simplex virus type-1 DNA polymerase and DNase. Eur J Biochem. 1990 Jul 31;191(2):263–273. doi: 10.1111/j.1432-1033.1990.tb19119.x. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Darby G. Related functional domains in virus DNA polymerases. EMBO J. 1987 Jan;6(1):169–175. doi: 10.1002/j.1460-2075.1987.tb04735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler E., Stuart A. D., Chee M. S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992 Jul 9;358(6382):160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Iltis J. P., Rapp F. Differential effect of arabinofuranosylthymine of the replication of human herpesviruses. J Virol. 1977 Sep;23(3):679–684. doi: 10.1128/jvi.23.3.679-684.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A., Bell J. B., Kunkel T. A., Sugino A. Eukaryotic DNA polymerase amino acid sequence required for 3'----5' exonuclease activity. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9473–9477. doi: 10.1073/pnas.88.21.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y., Maeno K., Yoshida S. Characterization of human cytomegalovirus-induced DNA polymerase and the associated 3'-to-5', exonuclease. Virology. 1983 Jan 30;124(2):221–231. doi: 10.1016/0042-6822(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Oberg B. Antiviral effects of phosphonoformate (PFA, foscarnet sodium). Pharmacol Ther. 1989;40(2):213–285. doi: 10.1016/0163-7258(89)90097-1. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Park J. B., Jay G. Neoplastic transformation of human keratinocytes by polybrene-induced DNA-mediated transfer of an activated oncogene. Oncogene. 1989 Nov;4(11):1403–1409. [PubMed] [Google Scholar]
- Shigeta S., Konno K., Baba M., Yokota T., De Clercq E. Comparative inhibitory effects of nucleoside analogues on different clinical isolates of human cytomegalovirus in vitro. J Infect Dis. 1991 Feb;163(2):270–275. doi: 10.1093/infdis/163.2.270. [DOI] [PubMed] [Google Scholar]
- Smee D. F., Boehme R., Chernow M., Binko B. P., Matthews T. R. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem Pharmacol. 1985 Apr 1;34(7):1049–1056. doi: 10.1016/0006-2952(85)90608-2. [DOI] [PubMed] [Google Scholar]
- Stanat S. C., Reardon J. E., Erice A., Jordan M. C., Drew W. L., Biron K. K. Ganciclovir-resistant cytomegalovirus clinical isolates: mode of resistance to ganciclovir. Antimicrob Agents Chemother. 1991 Nov;35(11):2191–2197. doi: 10.1128/aac.35.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan V., Coen D. M. Isolation of foscarnet-resistant human cytomegalovirus patterns of resistance and sensitivity to other antiviral drugs. J Infect Dis. 1991 Oct;164(4):781–784. doi: 10.1093/infdis/164.4.781. [DOI] [PubMed] [Google Scholar]
- Sullivan V., Talarico C. L., Stanat S. C., Davis M., Coen D. M., Biron K. K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992 Jul 9;358(6382):162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- Tatarowicz W. A., Lurain N. S., Thompson K. D. In situ ELISA for the evaluation of antiviral compounds effective against human cytomegalovirus. J Virol Methods. 1991 Nov-Dec;35(2):207–215. doi: 10.1016/0166-0934(91)90136-n. [DOI] [PubMed] [Google Scholar]
- Teo I. A., Griffin B. E., Jones M. D. Characterization of the DNA polymerase gene of human herpesvirus 6. J Virol. 1991 Sep;65(9):4670–4680. doi: 10.1128/jvi.65.9.4670-4680.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi T. Characterization of 3'-to 5'-exonuclease activity associated with Epstein-Barr virus DNA polymerase. Virology. 1991 May;182(1):376–381. doi: 10.1016/0042-6822(91)90685-5. [DOI] [PubMed] [Google Scholar]
- Wang Y. S., Woodward S., Hall J. D. Use of suppressor analysis to identify DNA polymerase mutations in herpes simplex virus which affect deoxynucleoside triphosphate substrate specificity. J Virol. 1992 Mar;66(3):1814–1816. doi: 10.1128/jvi.66.3.1814-1816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. W., Wahl A. F., Yuan P. M., Arai N., Pearson B. E., Arai K., Korn D., Hunkapiller M. W., Wang T. S. Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 1988 Jan;7(1):37–47. doi: 10.1002/j.1460-2075.1988.tb02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




