Abstract
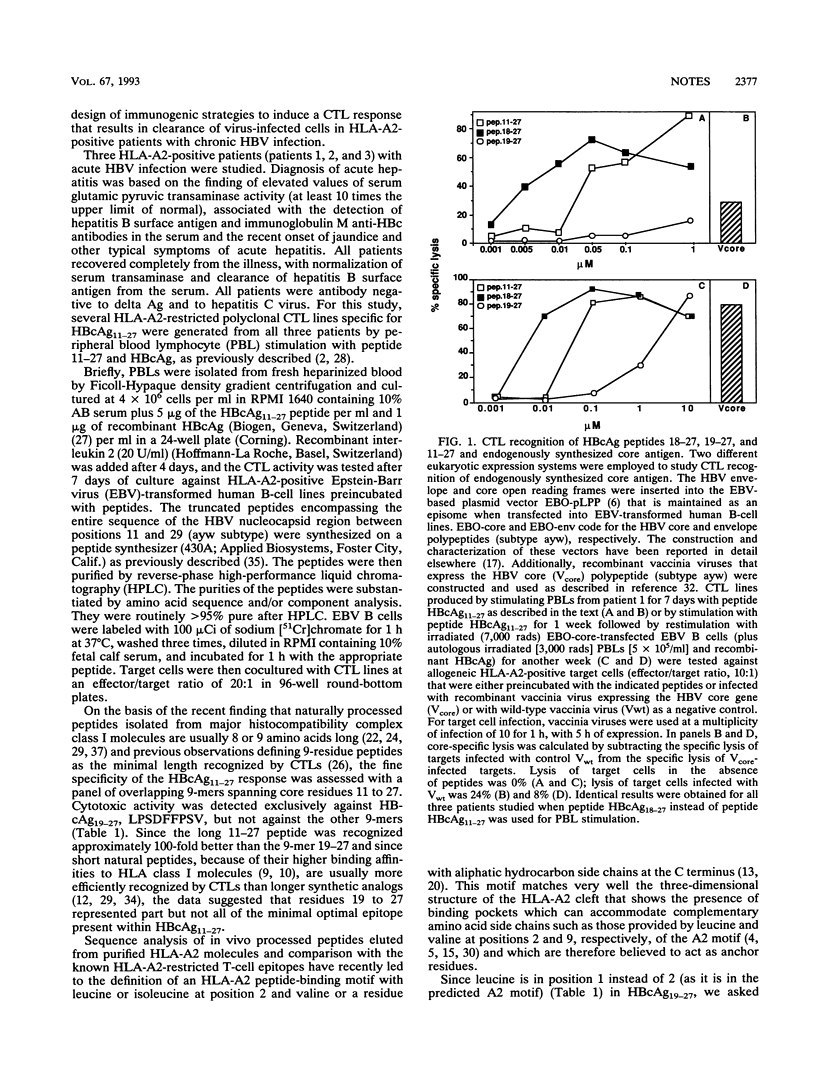
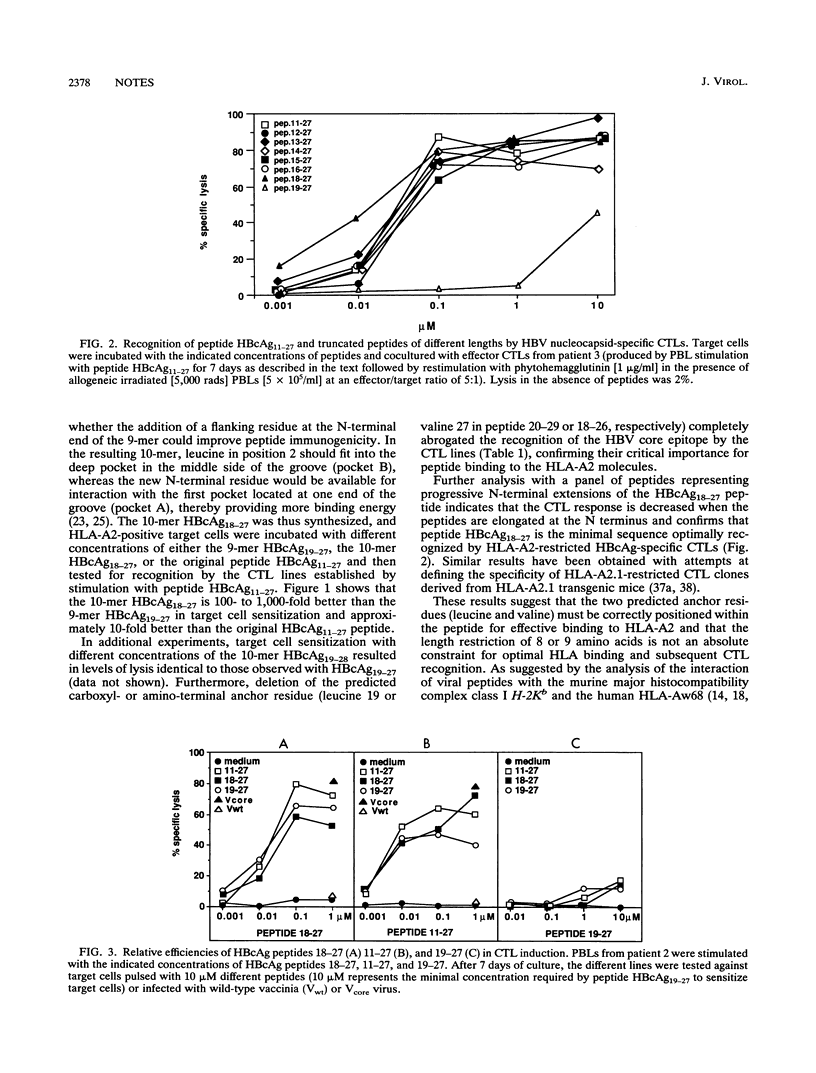
Residues 11 to 27 of the hepatitis B virus nucleocapsid antigen contain a cytotoxic T-cell epitope that is recognized by cytotoxic T cells from virtually all HLA-A2-positive patients with acute hepatitis B virus infection. Using panels of truncated and overlapping peptides, we now show that the optimal amino acid sequence recognized by cytotoxic T cells is a 10-mer (residues 18 to 27) containing the predicted peptide-binding motif for HLA-A2 and that this peptide can stimulate cytotoxic T cells able to recognize endogenously synthesized hepatitis B core antigen. Since patients with chronic hepatitis B virus infection fail to mount an efficient cytotoxic T-cell response to it, this epitope might serve as the starting point for the design of synthetic peptide-based immunotherapeutic strategies to terminate persistent viral infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aichele P., Hengartner H., Zinkernagel R. M., Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J Exp Med. 1990 May 1;171(5):1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A., Ferrari C., Fiaccadori F., Penna A., Margolskee R., Schlicht H. J., Fowler P., Guilhot S., Chisari F. V. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10445–10449. doi: 10.1073/pnas.88.23.10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Parham P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Canfield V., Emanuel J. R., Spickofsky N., Levenson R., Margolskee R. F. Ouabain-resistant mutants of the rat Na,K-ATPase alpha 2 isoform identified by using an episomal expression vector. Mol Cell Biol. 1990 Apr;10(4):1367–1372. doi: 10.1128/mcb.10.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F. R., Bevan M. J. Induction of ovalbumin-specific cytotoxic T cells by in vivo peptide immunization. J Exp Med. 1989 Mar 1;169(3):603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F. R., Moore M. W., Sheil J. M., Bevan M. J. Induction of cytotoxic T lymphocytes by primary in vitro stimulation with peptides. J Exp Med. 1988 Jun 1;167(6):1767–1779. doi: 10.1084/jem.167.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerundolo V., Elliott T., Elvin J., Bastin J., Rammensee H. G., Townsend A. The binding affinity and dissociation rates of peptides for class I major histocompatibility complex molecules. Eur J Immunol. 1991 Sep;21(9):2069–2075. doi: 10.1002/eji.1830210915. [DOI] [PubMed] [Google Scholar]
- Christinck E. R., Luscher M. A., Barber B. H., Williams D. B. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature. 1991 Jul 4;352(6330):67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Deres K., Metzger J., Jung G., Rammensee H. G. Identification of naturally processed viral nonapeptides allows their quantification in infected cells and suggests an allele-specific T cell epitope forecast. J Exp Med. 1991 Aug 1;174(2):425–434. doi: 10.1084/jem.174.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Garrett T. P., Saper M. A., Bjorkman P. J., Strominger J. L., Wiley D. C. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature. 1989 Dec 7;342(6250):692–696. doi: 10.1038/342692a0. [DOI] [PubMed] [Google Scholar]
- Gotch F., Rothbard J., Howland K., Townsend A., McMichael A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. 1987 Apr 30-May 6Nature. 326(6116):881–882. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- Guilhot S., Fowler P., Portillo G., Margolskee R. F., Ferrari C., Bertoletti A., Chisari F. V. Hepatitis B virus (HBV)-specific cytotoxic T-cell response in humans: production of target cells by stable expression of HBV-encoded proteins in immortalized human B-cell lines. J Virol. 1992 May;66(5):2670–2678. doi: 10.1128/jvi.66.5.2670-2678.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H. C., Jardetzky T. S., Garrett T. P., Lane W. S., Strominger J. L., Wiley D. C. Different length peptides bind to HLA-Aw68 similarly at their ends but bulge out in the middle. Nature. 1992 Nov 26;360(6402):364–366. doi: 10.1038/360364a0. [DOI] [PubMed] [Google Scholar]
- Harty J. T., Bevan M. J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992 Jun 1;175(6):1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. F., Henderson R. A., Shabanowitz J., Sakaguchi K., Michel H., Sevilir N., Cox A. L., Appella E., Engelhard V. H. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992 Mar 6;255(5049):1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- Ishioka G. Y., Colon S., Miles C., Grey H. M., Chesnut R. W. Induction of class I MHC-restricted, peptide-specific cytolytic T lymphocytes by peptide priming in vivo. J Immunol. 1989 Aug 15;143(4):1094–1100. [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Latron F., Pazmany L., Morrison J., Moots R., Saper M. A., McMichael A., Strominger J. L. A critical role for conserved residues in the cleft of HLA-A2 in presentation of a nonapeptide to T cells. Science. 1992 Aug 14;257(5072):964–967. doi: 10.1126/science.1380181. [DOI] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991 Sep 26;353(6342):321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Fremont D. H., Peterson P. A., Wilson I. A. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992 Aug 14;257(5072):927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Whitton J. L., Lewicki H., Tishon A. Fine dissection of a nine amino acid glycoprotein epitope, a major determinant recognized by lymphocytic choriomeningitis virus-specific class I-restricted H-2Db cytotoxic T lymphocytes. J Exp Med. 1988 Aug 1;168(2):559–570. doi: 10.1084/jem.168.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Penna A., Chisari F. V., Bertoletti A., Missale G., Fowler P., Giuberti T., Fiaccadori F., Ferrari C. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J Exp Med. 1991 Dec 1;174(6):1565–1570. doi: 10.1084/jem.174.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Saper M. A., Bjorkman P. J., Wiley D. C. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991 May 20;219(2):277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- Schild H., Norda M., Deres K., Falk K., Rötzschke O., Wiesmüller K. H., Jung G., Rammensee H. G. Fine specificity of cytotoxic T lymphocytes primed in vivo either with virus or synthetic lipopeptide vaccine or primed in vitro with peptide. J Exp Med. 1991 Dec 1;174(6):1665–1668. doi: 10.1084/jem.174.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Schaller H. The secretory core protein of human hepatitis B virus is expressed on the cell surface. J Virol. 1989 Dec;63(12):5399–5404. doi: 10.1128/jvi.63.12.5399-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M., Zinkernagel R. M., Hengartner H. Peptide-induced antiviral protection by cytotoxic T cells. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):991–993. doi: 10.1073/pnas.88.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher T. N., De Bruijn M. L., Vernie L. N., Kast W. M., Melief C. J., Neefjes J. J., Ploegh H. L. Peptide selection by MHC class I molecules. Nature. 1991 Apr 25;350(6320):703–706. doi: 10.1038/350703a0. [DOI] [PubMed] [Google Scholar]
- Sette A., Buus S., Colon S., Miles C., Grey H. M. Structural analysis of peptides capable of binding to more than one Ia antigen. J Immunol. 1989 Jan 1;142(1):35–40. [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Vitiello A., Marchesini D., Furze J., Sherman L. A., Chesnut R. W. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J Exp Med. 1991 Apr 1;173(4):1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]



