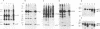Abstract
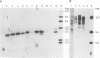
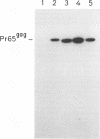
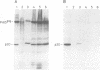
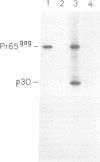
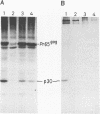
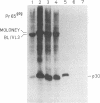
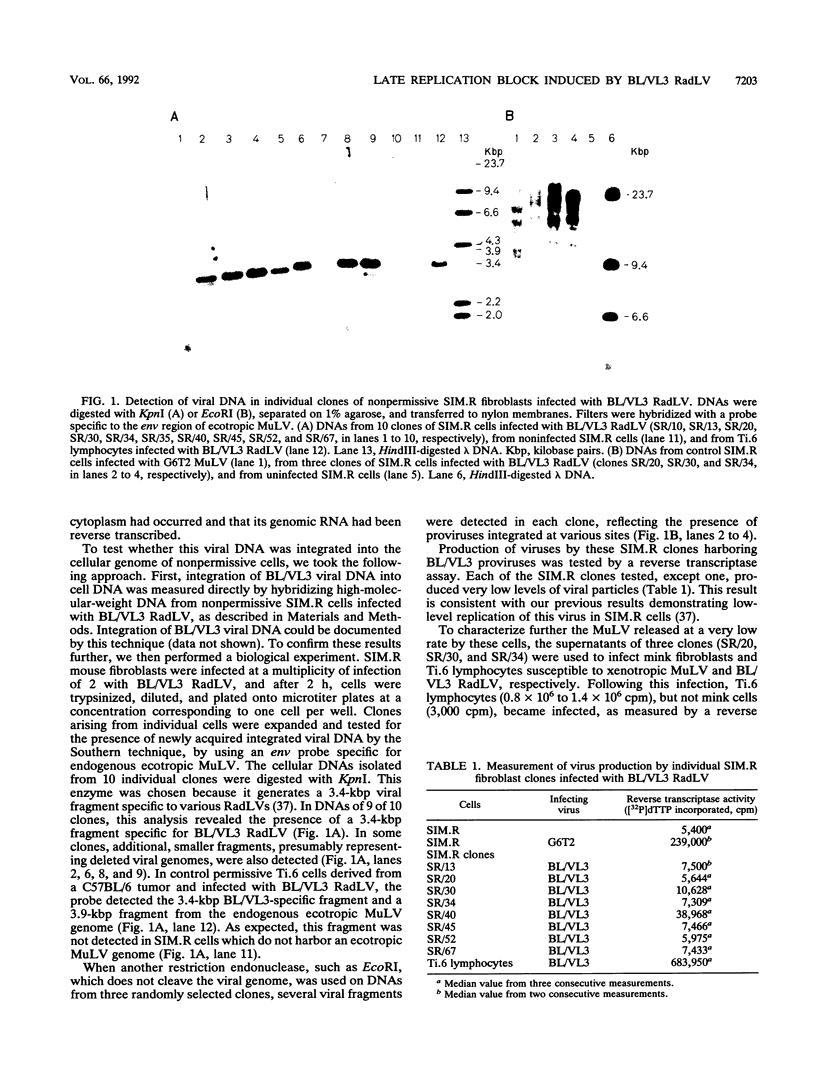
BL/VL3 radiation leukemia virus (RadLV) is a thymotropic, highly leukemogenic murine leukemia virus (MuLV) which is unable to replicate in vitro in mouse fibroblasts. We have previously reported that the U3 long terminal repeat region of its genome is responsible for this block (E. Rassart, Y. Paquette, and P. Jolicoeur, J. Virol. 62:3840-3848, 1988). By using hybrids of permissive and resistant cells infected with BL/VL3 RadLV or fibrotropic MuLV, we found that the resistant phenotype was dominant. Investigation to determine at which step of the virus cycle the block operates revealed that integration, transcription, and translation of the BL/VL3 viral genome occurred at normal levels in nonpermissive cells. The BL/VL3 RadLV Pr65gag proteins made in nonpermissive cells were also myristylated and located at the membrane, and the levels of their cleaved products were similar to those of fibrotropic MuLV. However, processing of BL/VL3 RadLV Pr85env was impaired in nonpermissive cells. Virions were not released into the culture medium of nonpermissive cells, as measured by reverse transcriptase activity and by content in p30 or gp70 protein and as documented by lower levels of budding particles seen by electron microscopy. These results indicate that BL/VL3 RadLV replication is blocked at a late stage of the virus cycle, i.e., at virion assembly. Interestingly, these BL/VL3 RadLV-infected nonpermissive fibroblasts were resistant to superinfection by fibrotropic Moloney MuLV, and this resistance also occurred at a late step of the Moloney virus cycle. Since this block is dominant, it appears that the U3 long terminal repeat region of the BL/VL3 viral genome has the ability to induce a cellular suppressor factor(s), thus bringing intracellular immunity against itself and against other ecotropic MuLVs.
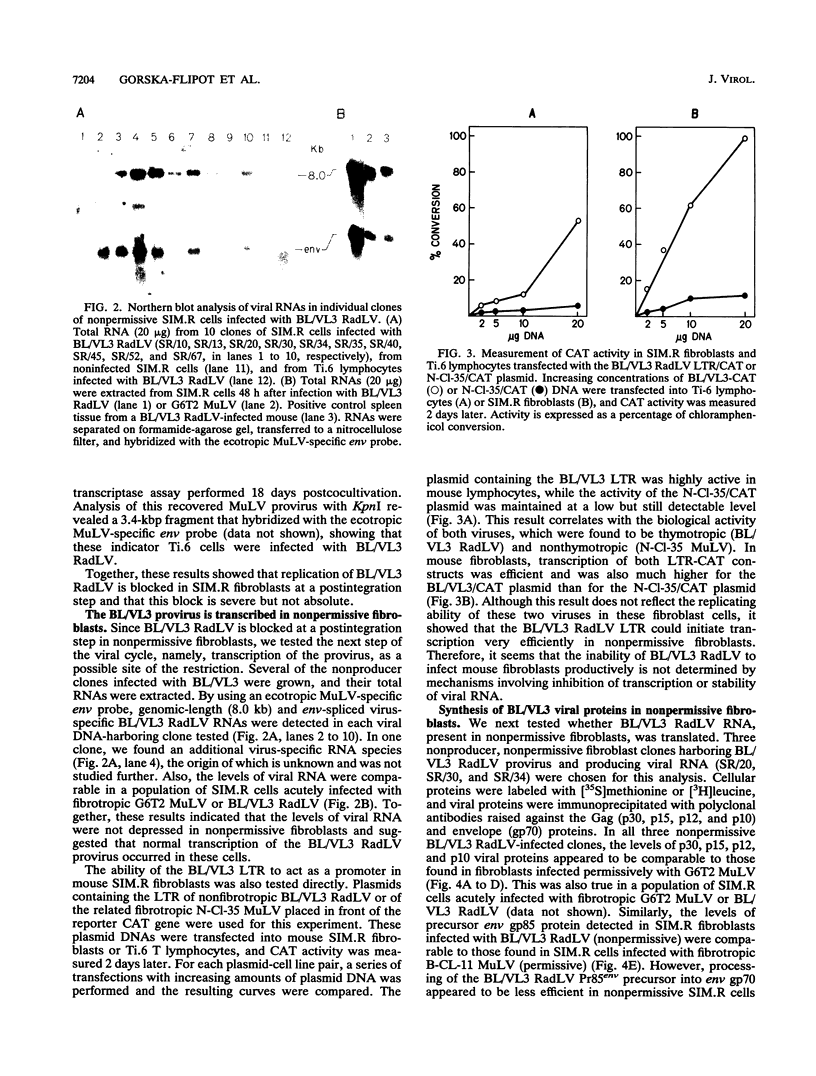
Full text
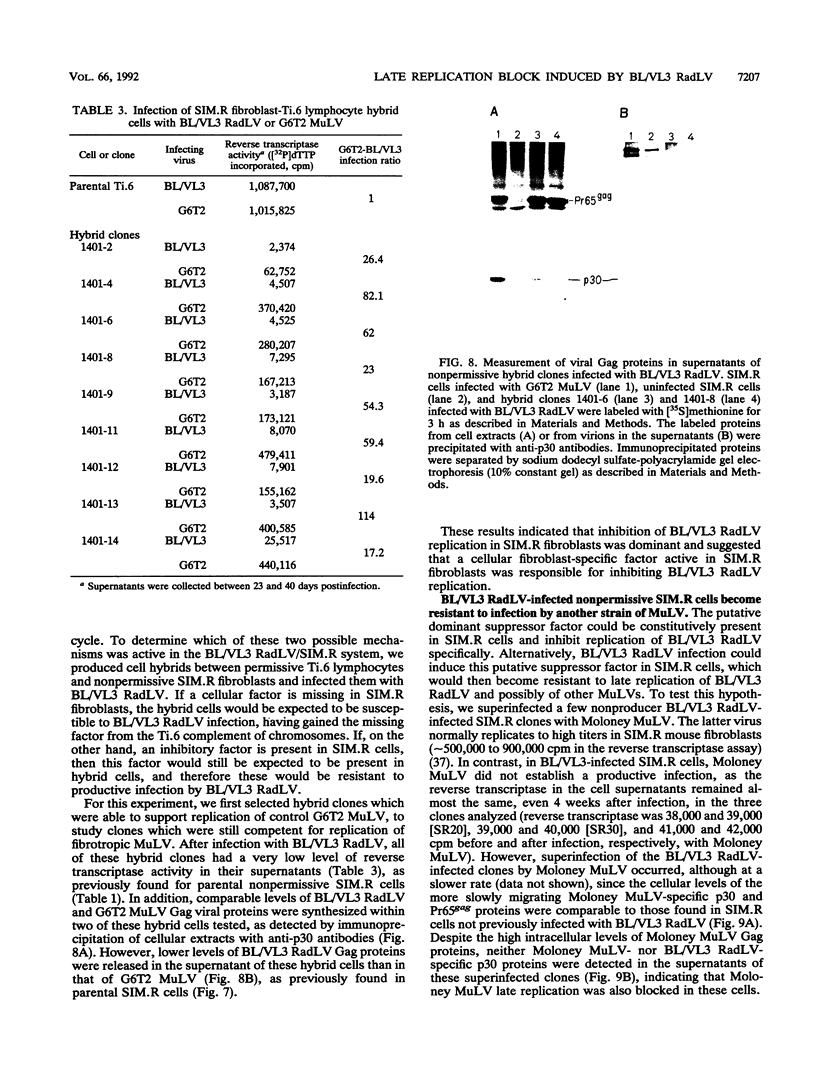
PDF

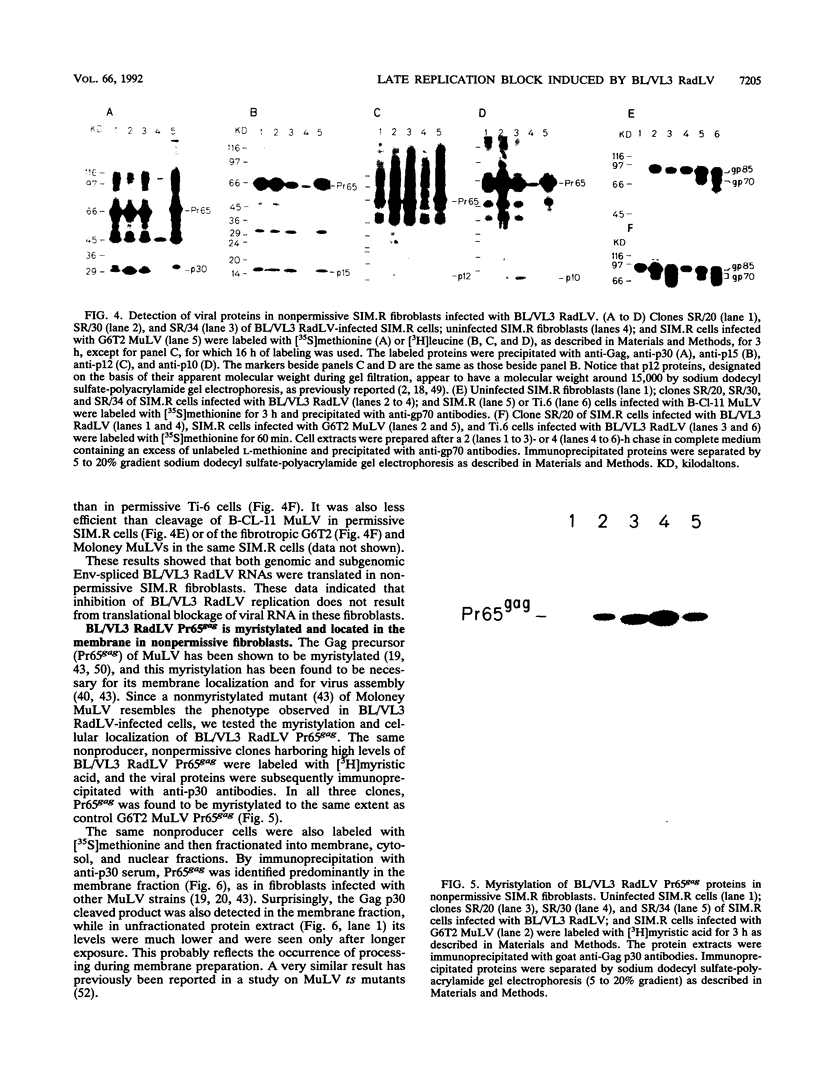



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Skuntz S., Noteborn M., Chang S., Meier E. Transgenic mice with intracellular immunity to influenza virus. Cell. 1990 Jul 13;62(1):51–61. doi: 10.1016/0092-8674(90)90239-b. [DOI] [PubMed] [Google Scholar]
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
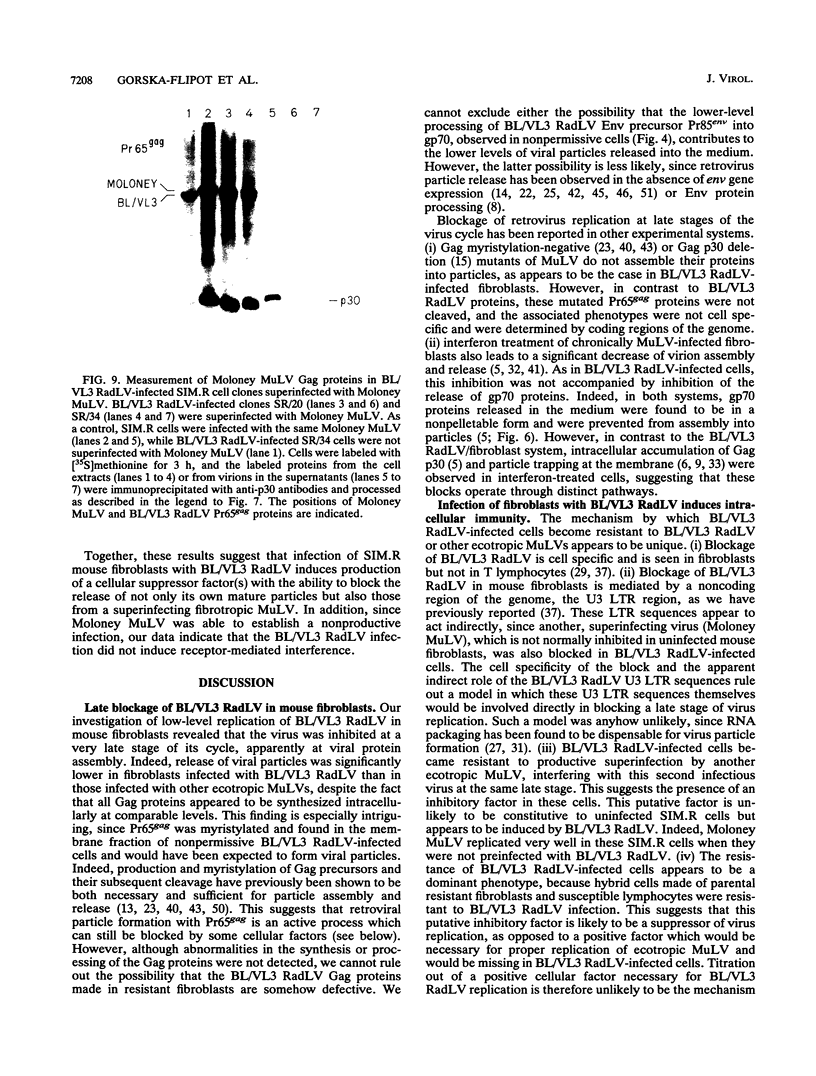
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988 Sep 29;335(6189):395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- Bilello J. A., Wivel N. A., Pitha P. M. Effect of interferon on the replication of mink cell focus-inducing virus in murine cells: synthesis, processing, assembly, and release of viral proteins. J Virol. 1982 Jul;43(1):213–222. doi: 10.1128/jvi.43.1.213-222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A., Heremans H., Allen P. T., De Maeyer-Guignard J., De Somer P. Trapping of oncornavirus particles at the surface of interferon-treated cells. Virology. 1976 Sep;73(2):537–542. doi: 10.1016/0042-6822(76)90416-5. [DOI] [PubMed] [Google Scholar]
- Bouchard L., Lamarre L., Tremblay P. J., Jolicoeur P. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell. 1989 Jun 16;57(6):931–936. doi: 10.1016/0092-8674(89)90331-0. [DOI] [PubMed] [Google Scholar]
- Bour S., Boulerice F., Wainberg M. A. Inhibition of gp160 and CD4 maturation in U937 cells after both defective and productive infections by human immunodeficiency virus type 1. J Virol. 1991 Dec;65(12):6387–6396. doi: 10.1128/jvi.65.12.6387-6396.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Mims S. J., Triche T. J., Friedman R. M. Interferon inhibits mouse leukaemia virus release: an electron microscope study. J Gen Virol. 1977 Feb;34(2):363–367. doi: 10.1099/0022-1317-34-2-363. [DOI] [PubMed] [Google Scholar]
- Choi Y., Kappler J. W., Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991 Mar 21;350(6315):203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Declève A., Sato C., Lieberman M., Kaplan H. S. Selective thymic localization of murine leukemia virus-related antigens in C57BL-Ka mice after inoculation with radiation virus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3124–3128. doi: 10.1073/pnas.71.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D., De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989 Oct 6;59(1):103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Lobel L. I. Mutants of murine leukemia viruses and retroviral replication. Biochim Biophys Acta. 1987 Jul 8;907(2):93–123. doi: 10.1016/0304-419x(87)90001-1. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green R. W., Bolognesi D. P., Schäfer W., Pister L., Hunsmann G., De Noronha F. Polypeptides of mammalian oncornaviruses. I. Isolation and serological analysis polypeptides from murine and feline C-type viruses. Virology. 1973 Dec;56(2):565–579. doi: 10.1016/0042-6822(73)90058-5. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Jolicoeur P. Characterization of the gag/fusion protein encoded by the defective Duplan retrovirus inducing murine acquired immunodeficiency syndrome. J Virol. 1990 Dec;64(12):5764–5772. doi: 10.1128/jvi.64.12.5764-5772.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J Virol. 1980 Jan;33(1):183–195. doi: 10.1128/jvi.33.1.183-195.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Blaug G., Hansen M., Barklis E. Assembly of gag-beta-galactosidase proteins into retrovirus particles. J Virol. 1990 May;64(5):2265–2279. doi: 10.1128/jvi.64.5.2265-2279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen E. C., Kjeldgaard N. O., Pedersen F. S., Jørgensen P. A nucleotide substitution in the gag N terminus of the endogenous ecotropic DBA/2 virus prevents Pr65gag myristylation and virus replication. J Virol. 1988 Sep;62(9):3217–3223. doi: 10.1128/jvi.62.9.3217-3223.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Karacostas V., Nagashima K., Gonda M. A., Moss B. Human immunodeficiency virus-like particles produced by a vaccinia virus expression vector. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8964–8967. doi: 10.1073/pnas.86.22.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Grimley P. M., Ramseur J. M., Berezesky I. K. Deficiency of 60 to 70S RNA in murine leukemia virus particles assembled in cells treated with actinomycin D. J Virol. 1974 Jul;14(1):152–161. doi: 10.1128/jvi.14.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Declève A., Kaplan H. S. Rapid in vitro assay for thymotropic, leukemogenic murine C-type RNA viruses. Virology. 1978 Oct 15;90(2):274–278. doi: 10.1016/0042-6822(78)90311-2. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Niwa O., Declève A., Kaplan H. S. Continuous propagation of radiation leukemia virus on a C57BL mouse-embryo fibroblast line, with attenuation of leukemogenic activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1250–1253. doi: 10.1073/pnas.70.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Staal S. P., Bolognesi D. P., Denny T. P., Rowe W. P. Effect of interferon on murine leukemia virus infection. II. Synthesis of viral components in exogenous infection. Virology. 1977 Jun 1;79(1):1–13. doi: 10.1016/0042-6822(77)90329-4. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Wivel N. A., Fernie B. F., Harper H. P. Effect of interferon on murine leukaemia virus infection. IV. Formation of non-infectious virus in chronically infected cells. J Gen Virol. 1979 Mar;42(3):467–480. doi: 10.1099/0022-1317-42-3-467. [DOI] [PubMed] [Google Scholar]
- Poirier Y., Kozak C., Jolicoeur P. Identification of a common helper provirus integration site in Abelson murine leukemia virus-induced lymphoma DNA. J Virol. 1988 Nov;62(11):3985–3992. doi: 10.1128/jvi.62.11.3985-3992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Rassart E., DesGroseillers L., Jolicoeur P. Molecular cloning of B- and N-tropic endogenous BALB/c murine leukemia virus circular DNA intermediates: isolation and characterization of infectious recombinant clones. J Virol. 1981 Jul;39(1):162–171. doi: 10.1128/jvi.39.1.162-171.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., Paquette Y., Jolicoeur P. Inability of Kaplan radiation leukemia virus to replicate on mouse fibroblasts is conferred by its long terminal repeat. J Virol. 1988 Oct;62(10):3840–3848. doi: 10.1128/jvi.62.10.3840-3848.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., Sankar-Mistry P., Lemay G., DesGroseillers L., Jolicoeur P. New class of leukemogenic ecotropic recombinant murine leukemia virus isolated from radiation-induced thymomas of C57BL/6 mice. J Virol. 1983 Feb;45(2):565–575. doi: 10.1128/jvi.45.2.565-575.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., Shang M., Boie Y., Jolicoeur P. Studies on emerging radiation leukemia virus variants in C57BL/Ka mice. J Virol. 1986 Apr;58(1):96–106. doi: 10.1128/jvi.58.1.96-106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg S., Heller A., Aboud M., Gurari-Rotman D., Revel M. Effect of interferon on human cells releasing oncornaviruses: an assay for human interferon. Virology. 1979 Feb;93(1):209–214. doi: 10.1016/0042-6822(79)90288-5. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Rein A. Unmyristylated Moloney murine leukemia virus Pr65gag is excluded from virus assembly and maturation events. J Virol. 1989 May;63(5):2370–2373. doi: 10.1128/jvi.63.5.2370-2373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., De Maeyer E., Riviere I., De Maeyer-Guignard J. Stable antiviral expression in BALB/c 3T3 cells carrying a beta interferon sequence behind a major histocompatibility complex promoter fragment. J Virol. 1991 Feb;65(2):664–671. doi: 10.1128/jvi.65.2.664-671.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Cho M. I., Hammarskjöld M. L., Rekosh D. Human immunodeficiency virus type 1 Pr55gag and Pr160gag-pol expressed from a simian virus 40 late replacement vector are efficiently processed and assembled into viruslike particles. J Virol. 1990 Jun;64(6):2743–2750. doi: 10.1128/jvi.64.6.2743-2750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola C., Thibault G., Haile-Meskel H., Anand-Srivastava M. B., Garcia R., Cantin M. Atrial natriuretic factor in the vena cava and sinus node. J Histochem Cytochem. 1990 Aug;38(8):1123–1135. doi: 10.1177/38.8.2142177. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Haller O., Boll W., Lindenmann J., Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986 Jan 17;44(1):147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Tronick S. R., Aaronson S. A. Analysis of type specific antigenic determinants of two structural polypeptides of mouse RNA C-Type viruses. Virology. 1974 Mar;58(1):1–8. doi: 10.1016/0042-6822(74)90135-4. [DOI] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Achacoso J. A. Creation and expression of myristylated forms of Rous sarcoma virus gag protein in mammalian cells. J Virol. 1989 Oct;63(10):4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbl H., Latreille J., Jolicoeur P. Revertants of v-fos-transformed fibroblasts have mutations in cellular genes essential for transformation by other oncogenes. Cell. 1987 Nov 6;51(3):357–369. doi: 10.1016/0092-8674(87)90632-5. [DOI] [PubMed] [Google Scholar]