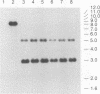
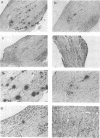
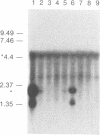
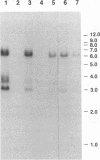
Abstract
Ocular infection of immunocompetent (BALB/c) mice with wild-type herpes simplex virus type 1 (HSV-1) 17+ may lead to acute fatal encephalitis; however, in surviving animals, a latent (nonproductive) infection of the nervous system is established. In contrast, 17+ infection invariably kills mice with severe combined immunodeficiency (SCID mice) within 2 weeks. Ocular infection of immunocompetent mice with a mutant HSV-1 strain, in1814, which does not produce a functional alpha-transinducing protein, results in no detectable viral replication in the nervous system during the time corresponding to the acute phase of infection, no mortality, and the establishment of latency. In SCID mice, however, the in1814 virus establishes a unique, slowly progressing infection. In studying the courses of in1814 infection in SCID and BALB/c mice, we found that although intact B- and/or T-lymphocytic functions were required for the control of viral replication in the nervous system, some of the infected neurons of SCID mice seemed to be able to restrict in1814 replication and harbor the virus in a latent state.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ace C. I., McKee T. A., Ryan J. M., Cameron J. M., Preston C. M. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J Virol. 1989 May;63(5):2260–2269. doi: 10.1128/jvi.63.5.2260-2269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bosma M. J., Carroll A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Devi B. G., Anderson K. P., Gaylord B. H., Wagner E. K. Characterization of a major late herpes simplex virus type 1 mRNA. J Virol. 1981 May;38(2):483–496. doi: 10.1128/jvi.38.2.483-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. B., Taylor J. A., Oakes J. E. Ocular infection with herpes simplex virus type 1: prevention of acute herpetic encephalitis by systemic administration of virus-specific antibody. J Infect Dis. 1979 Oct;140(4):534–540. doi: 10.1093/infdis/140.4.534. [DOI] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., Fraser N. W. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987 May;84(10):3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., O'Boyle D. R., 2nd, Fraser N. W. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J Virol. 1988 Mar;62(3):749–756. doi: 10.1128/jvi.62.3.749-756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane S. L., Fraser N. W. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J Virol. 1989 Feb;63(2):943–947. doi: 10.1128/jvi.63.2.943-947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou S., Minson A. C., Field H. J., Anderson J. R., Wildy P. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in humans. J Virol. 1986 Feb;57(2):446–455. doi: 10.1128/jvi.57.2.446-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N. W., Spivack J. G., Wroblewska Z., Block T., Deshmane S. L., Valyi-Nagy T., Natarajan R., Gesser R. M. A review of the molecular mechanism of HSV-1 latency. Curr Eye Res. 1991;10 (Suppl):1–13. doi: 10.3109/02713689109020352. [DOI] [PubMed] [Google Scholar]
- Frink R. J., Anderson K. P., Wagner E. K. Herpes simplex virus type 1 HindIII fragment L encodes spliced and complementary mRNA species. J Virol. 1981 Aug;39(2):559–572. doi: 10.1128/jvi.39.2.559-572.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Blanco M. A., Cullen B. R. Molecular basis of latency in pathogenic human viruses. Science. 1991 Nov 8;254(5033):815–820. doi: 10.1126/science.1658933. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A. K., Nash A. A., Wildy P. Pathogenesis of herpes simplex virus in B cell-suppressed mice: the relative roles of cell-mediated and humoral immunity. J Gen Virol. 1982 Jul;61(Pt 50):127–131. doi: 10.1099/0022-1317-61-1-127. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Dent C. L., Latchman D. S. Octamer motif mediates transcriptional repression of HSV immediate-early genes and octamer-containing cellular promoters in neuronal cells. Neuron. 1990 Feb;4(2):215–222. doi: 10.1016/0896-6273(90)90096-x. [DOI] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Latent herpes simplex virus in the central nervous system of rabbits and mice. J Exp Med. 1973 Sep 1;138(3):740–744. doi: 10.1084/jem.138.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Pathogenesis of herpetic encephalitis in mice after ophthalmic inoculation. J Infect Dis. 1974 Jul;130(1):16–27. doi: 10.1093/infdis/130.1.16. [DOI] [PubMed] [Google Scholar]
- Margolis T. P., Dawson C. R., LaVail J. H. Herpes simplex viral infection of the mouse trigeminal ganglion. Immunohistochemical analysis of cell populations. Invest Ophthalmol Vis Sci. 1992 Feb;33(2):259–267. [PubMed] [Google Scholar]
- Mellerick D. M., Fraser N. W. Physical state of the latent herpes simplex virus genome in a mouse model system: evidence suggesting an episomal state. Virology. 1987 Jun;158(2):265–275. doi: 10.1016/0042-6822(87)90198-x. [DOI] [PubMed] [Google Scholar]
- Minagawa H., Sakuma S., Mohri S., Mori R., Watanabe T. Herpes simplex virus type 1 infection in mice with severe combined immunodeficiency (SCID). Arch Virol. 1988;103(1-2):73–82. doi: 10.1007/BF01319810. [DOI] [PubMed] [Google Scholar]
- Mori R., Minagawa H., Sakuma S., Mohri S., Watanabe T. Herpes simplex virus type 1 infection in mice with severe combined immunodeficiency (SCID). Adv Exp Med Biol. 1990;278:191–197. doi: 10.1007/978-1-4684-5853-4_19. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Oda H., Mori R., Taniguchi T. Mechanism of acquired resistance to herpes simplex virus infection as studied in nude mice. J Gen Virol. 1979 Sep;44(3):715–723. doi: 10.1099/0022-1317-44-3-715. [DOI] [PubMed] [Google Scholar]
- Oakes J. E., Lausch R. N. Monoclonal antibodies suppress replication of herpes simplex virus type 1 in trigeminal ganglia. J Virol. 1984 Sep;51(3):656–661. doi: 10.1128/jvi.51.3.656-661.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular anatomy of viral persistence. J Virol. 1991 Dec;65(12):6381–6386. doi: 10.1128/jvi.65.12.6381-6386.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw H., Asher L. V., Wohlenberg C., Sekizawa T., Notkins A. L. Acute and latent infection of sensory ganglia with herpes simplex virus: immune control and virus reactivation. J Gen Virol. 1979 Jul;44(1):205–215. doi: 10.1099/0022-1317-44-1-205. [DOI] [PubMed] [Google Scholar]
- Poffenberger K. L., Roizman B. A noninverting genome of a viable herpes simplex virus 1: presence of head-to-tail linkages in packaged genomes and requirements for circularization after infection. J Virol. 1985 Feb;53(2):587–595. doi: 10.1128/jvi.53.2.587-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Conley A. J., Mocarski E. S., Roizman B. Cloning of reiterated and nonreiterated herpes simplex virus 1 sequences as BamHI fragments. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4201–4205. doi: 10.1073/pnas.77.7.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983 Apr 7;302(5908):523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Latent herpes simplex virus type 1 DNA contains two copies of the virion DNA joint region. J Virol. 1985 Sep;55(3):849–852. doi: 10.1128/jvi.55.3.849-852.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Sears A. E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Sears A. E. Mechanisms of restriction of viral gene expression during herpes simplex virus latency. Adv Exp Med Biol. 1990;278:211–217. doi: 10.1007/978-1-4684-5853-4_22. [DOI] [PubMed] [Google Scholar]
- Shimeld C., Hill T. J., Blyth W. A., Easty D. L. Passive immunization protects the mouse eye from damage after herpes simplex virus infection by limiting spread of virus in the nervous system. J Gen Virol. 1990 Mar;71(Pt 3):681–687. doi: 10.1099/0022-1317-71-3-681. [DOI] [PubMed] [Google Scholar]
- Shimeld C., Hill T. J., Blyth W. A., Easty D. L. Reactivation of latent infection and induction of recurrent herpetic eye disease in mice. J Gen Virol. 1990 Feb;71(Pt 2):397–404. doi: 10.1099/0022-1317-71-2-397. [DOI] [PubMed] [Google Scholar]
- Simmons A., Tscharke D. C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992 May 1;175(5):1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Expression of herpes simplex virus type 1 latency-associated transcripts in the trigeminal ganglia of mice during acute infection and reactivation of latent infection. J Virol. 1988 May;62(5):1479–1485. doi: 10.1128/jvi.62.5.1479-1485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Deshmane S. L., Ace C. I., Preston C. M., Fraser N. W. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J Virol. 1990 Apr;64(4):1630–1638. doi: 10.1128/jvi.64.4.1630-1638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Lirette R. P., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989 Feb;8(2):505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989 Sep;53(3):318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Valyi-Nagy T., Deshmane S. L., Spivack J. G., Steiner I., Ace C. I., Preston C. M., Fraser N. W. Investigation of herpes simplex virus type 1 (HSV-1) gene expression and DNA synthesis during the establishment of latent infection by an HSV-1 mutant, in1814, that does not replicate in mouse trigeminal ganglia. J Gen Virol. 1991 Mar;72(Pt 3):641–649. doi: 10.1099/0022-1317-72-3-641. [DOI] [PubMed] [Google Scholar]
- Valyi-Nagy T., Deshmane S., Dillner A., Fraser N. W. Induction of cellular transcription factors in trigeminal ganglia of mice by corneal scarification, herpes simplex virus type 1 infection, and explantation of trigeminal ganglia. J Virol. 1991 Aug;65(8):4142–4152. doi: 10.1128/jvi.65.8.4142-4152.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Notkins A. L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974 Jun 14;184(4142):1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]
- Wilcox C. L., Johnson E. M., Jr Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J Virol. 1987 Jul;61(7):2311–2315. doi: 10.1128/jvi.61.7.2311-2315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey D. E., Trousdale M. D., Nesburn A. B. Reactivation of murine latent HSV infection by epinephrine iontophoresis. Invest Ophthalmol Vis Sci. 1984 Aug;25(8):945–950. [PubMed] [Google Scholar]
- Yao F., Courtney R. J. A major transcriptional regulatory protein (ICP4) of herpes simplex virus type 1 is associated with purified virions. J Virol. 1989 Aug;63(8):3338–3344. doi: 10.1128/jvi.63.8.3338-3344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]