Abstract
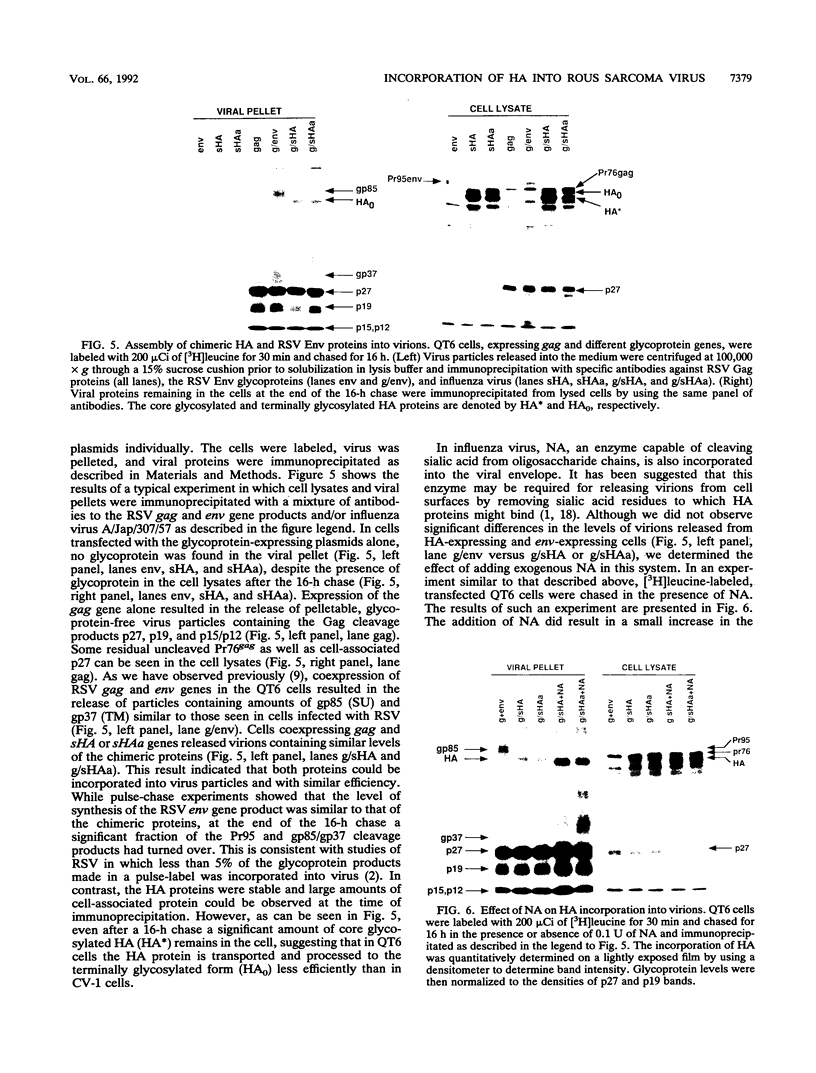
We have investigated what protein sequences are necessary for glycoprotein incorporation into Rous sarcoma virus (RSV) virions by utilizing the hemagglutinin (HA) protein of influenza virus. Two chimeric HA genes were constructed. In the first the coding sequence for the signal peptide of the RSV env gene product was fused in frame to the entire HA structural gene, and in the second the hydrophobic anchor and cytoplasmic domain sequences of the HA gene were also replaced with those from the RSV env gene. Both chimeric genes, expressed from a simian virus 40 expression vector in CV-1 cells, yielded functional HA proteins that were transported to the cell surface and were able to bind to erythrocytes. When the genes were expressed in combination with the RSV gag-pol gene region in QT6 cells by using a vaccinia virus-T7 expression/complementation system, virions that efficiently incorporated either chimeric protein were assembled. This result indicated that the presence of the RSV env membrane anchor and cytoplasmic sequences did not facilitate HA glycoprotein incorporation into virions. The presence of the RSV env signal sequence allowed the chimeric HA genes to be substituted into the RSV-derived BH-RCAN.HiSV viral genome in place of the RSV env gene. Both chimeric genomes yielded infectious virus that could infect human and avian cells with equal efficiency. These experiments demonstrate that a foreign glycoprotein, efficiently incorporated into virions lacking a native glycoprotein, can confer a broadened host range on the virus. Moreover, because the HA of influenza virus requires the acidic pH of the endosome in order to be activated, these results imply that foreign proteins can modify the normal route of entry of this avian retrovirus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basak S., Tomana M., Compans R. W. Sialic acid is incorporated into influenza hemagglutinin glycoproteins in the absence of viral neuraminidase. Virus Res. 1985 Feb;2(1):61–68. doi: 10.1016/0168-1702(85)90060-7. [DOI] [PubMed] [Google Scholar]
- Bosch J. V., Schwarz R. T., Ziemiecki A., Friis R. R. Oligosaccharide modifications and the site of processing of gPr92env, the precursor for the viral glycoproteins of Rous sarcoma virus. Virology. 1982 May;119(1):122–132. doi: 10.1016/0042-6822(82)90070-8. [DOI] [PubMed] [Google Scholar]
- Brody B. A., Rhee S. S., Sommerfelt M. A., Hunter E. A viral protease-mediated cleavage of the transmembrane glycoprotein of Mason-Pfizer monkey virus can be suppressed by mutations within the matrix protein. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3443–3447. doi: 10.1073/pnas.89.8.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. L., Hunter E. A charged amino acid substitution within the transmembrane anchor of the Rous sarcoma virus envelope glycoprotein affects surface expression but not intracellular transport. J Cell Biol. 1987 Sep;105(3):1191–1203. doi: 10.1083/jcb.105.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Helenius A. Quaternary structure of influenza virus hemagglutinin after acid treatment. J Virol. 1986 Dec;60(3):833–839. doi: 10.1128/jvi.60.3.833-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Helenius A., White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem. 1985 Mar 10;260(5):2973–2981. [PubMed] [Google Scholar]
- Dong J. Y., Dubay J. W., Perez L. G., Hunter E. Mutations within the proteolytic cleavage site of the Rous sarcoma virus glycoprotein define a requirement for dibasic residues for intracellular cleavage. J Virol. 1992 Feb;66(2):865–874. doi: 10.1128/jvi.66.2.865-874.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emi N., Friedmann T., Yee J. K. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol. 1991 Mar;65(3):1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt A., Bosch J. V., Ziemiecki A., Friis R. R. Rous sarcoma virus p19 and gp35 can be chemically crosslinked to high molecular weight complexes. An insight into virus assembly. J Mol Biol. 1984 Apr 5;174(2):297–317. doi: 10.1016/0022-2836(84)90340-1. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Cell-surface expression of influenza haemagglutinin from a cloned DNA copy of the RNA gene. Nature. 1981 Oct 22;293(5834):620–625. doi: 10.1038/293620a0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Construction of influenza haemagglutinin genes that code for intracellular and secreted forms of the protein. Nature. 1982 Dec 16;300(5893):598–603. doi: 10.1038/300598a0. [DOI] [PubMed] [Google Scholar]
- Greenhouse J. J., Petropoulos C. J., Crittenden L. B., Hughes S. H. Helper-independent retrovirus vectors with Rous-associated virus type O long terminal repeats. J Virol. 1988 Dec;62(12):4809–4812. doi: 10.1128/jvi.62.12.4809-4812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. A., Basak S., Compans R. W. Effects of hexose starvation and the role of sialic acid in influenza virus release. Virology. 1983 Mar;125(2):324–334. doi: 10.1016/0042-6822(83)90205-2. [DOI] [PubMed] [Google Scholar]
- Hughes S., Kosik E. Mutagenesis of the region between env and src of the SR-A strain of Rous sarcoma virus for the purpose of constructing helper-independent vectors. Virology. 1984 Jul 15;136(1):89–99. doi: 10.1016/0042-6822(84)90250-2. [DOI] [PubMed] [Google Scholar]
- Hunter E. Biological techniques for avian sarcoma viruses. Methods Enzymol. 1979;58:379–393. doi: 10.1016/s0076-6879(79)58153-1. [DOI] [PubMed] [Google Scholar]
- Kaster K. R., Burgett S. G., Rao R. N., Ingolia T. D. Analysis of a bacterial hygromycin B resistance gene by transcriptional and translational fusions and by DNA sequencing. Nucleic Acids Res. 1983 Oct 11;11(19):6895–6911. doi: 10.1093/nar/11.19.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. A., Omer C. A., Weis J. H., Mitsialis S. A., Faras A. J., Guntaka R. V. Restriction endonuclease and nucleotide sequence analyses of molecularly cloned unintegrated avian tumor virus DNA: structure of large terminal repeats in circle junctions. J Virol. 1982 Apr;42(1):346–351. doi: 10.1128/jvi.42.1.346-351.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau N. R., Page K. A., Littman D. R. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J Virol. 1991 Jan;65(1):162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovits J., Shia S. P., Ktistakis N., Lee M. S., Bird C., Roth M. G. The effects of foreign transmembrane domains on the biosynthesis of the influenza virus hemagglutinin. J Biol Chem. 1990 Mar 15;265(8):4760–4767. [PubMed] [Google Scholar]
- Leamnson R. N., Halpern M. S. Subunit structure of the glycoprotein complex of avian tumor virus. J Virol. 1976 Jun;18(3):956–968. doi: 10.1128/jvi.18.3.956-968.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. O., Sommerfelt M. A., Marsh M., Weiss R. A. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990 Apr;71(Pt 4):767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Garcia J. V., von Suhr N., Lynch C. M., Wilson C., Eiden M. V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991 May;65(5):2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Perez L. G., Davis G. L., Hunter E. Mutants of the Rous sarcoma virus envelope glycoprotein that lack the transmembrane anchor and cytoplasmic domains: analysis of intracellular transport and assembly into virions. J Virol. 1987 Oct;61(10):2981–2988. doi: 10.1128/jvi.61.10.2981-2988.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez L., Wills J. W., Hunter E. Expression of the Rous sarcoma virus env gene from a simian virus 40 late-region replacement vector: effects of upstream initiation codons. J Virol. 1987 Apr;61(4):1276–1281. doi: 10.1128/jvi.61.4.1276-1281.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos C. J., Hughes S. H. Replication-competent retrovirus vectors for the transfer and expression of gene cassettes in avian cells. J Virol. 1991 Jul;65(7):3728–3737. doi: 10.1128/jvi.65.7.3728-3737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. A single amino acid substitution within the matrix protein of a type D retrovirus converts its morphogenesis to that of a type C retrovirus. Cell. 1990 Oct 5;63(1):77–86. doi: 10.1016/0092-8674(90)90289-q. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Wade E., Wright D. A., Koval V., Clark C., Jaquish D., Spector S. A. Human immunodeficiency virus pseudotypes with expanded cellular and species tropism. J Virol. 1990 May;64(5):2298–2308. doi: 10.1128/jvi.64.5.2298-2308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Boettiger D., Murphy H. M. Pseudotypes of avian sarcoma viruses with the envelope properties of vesicular stomatitis virus. Virology. 1977 Feb;76(2):808–825. doi: 10.1016/0042-6822(77)90261-6. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Wong A. L. Phenotypic mixing between avian and mammalian RNA tumor viruses: I. Envelope pseudotypes of Rous sarcoma virus. Virology. 1977 Feb;76(2):826–834. doi: 10.1016/0042-6822(77)90262-8. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Hardwick J. M., Shaw K., Hunter E. Alterations in the transport and processing of Rous sarcoma virus envelope glycoproteins mutated in the signal and anchor regions. J Cell Biochem. 1983;23(1-4):81–94. doi: 10.1002/jcb.240230109. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Srinivas R. V., Hunter E. Mutations of the Rous sarcoma virus env gene that affect the transport and subcellular location of the glycoprotein products. J Cell Biol. 1984 Dec;99(6):2011–2023. doi: 10.1083/jcb.99.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C., Reitz M. S., Okayama H., Eiden M. V. Formation of infectious hybrid virions with gibbon ape leukemia virus and human T-cell leukemia virus retroviral envelope glycoproteins and the gag and pol proteins of Moloney murine leukemia virus. J Virol. 1989 May;63(5):2374–2378. doi: 10.1128/jvi.63.5.2374-2378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. A., Bates P., Willert K., Varmus H. E. Efficient incorporation of human CD4 protein into avian leukosis virus particles. Science. 1990 Dec 7;250(4986):1421–1423. doi: 10.1126/science.2175047. [DOI] [PubMed] [Google Scholar]
- de Giuli C., Kawai S., Dales S., Hanafusa H. Absence of surface projections of some noninfectious forms of RSV. Virology. 1975 Jul;66(1):253–260. doi: 10.1016/0042-6822(75)90195-6. [DOI] [PubMed] [Google Scholar]