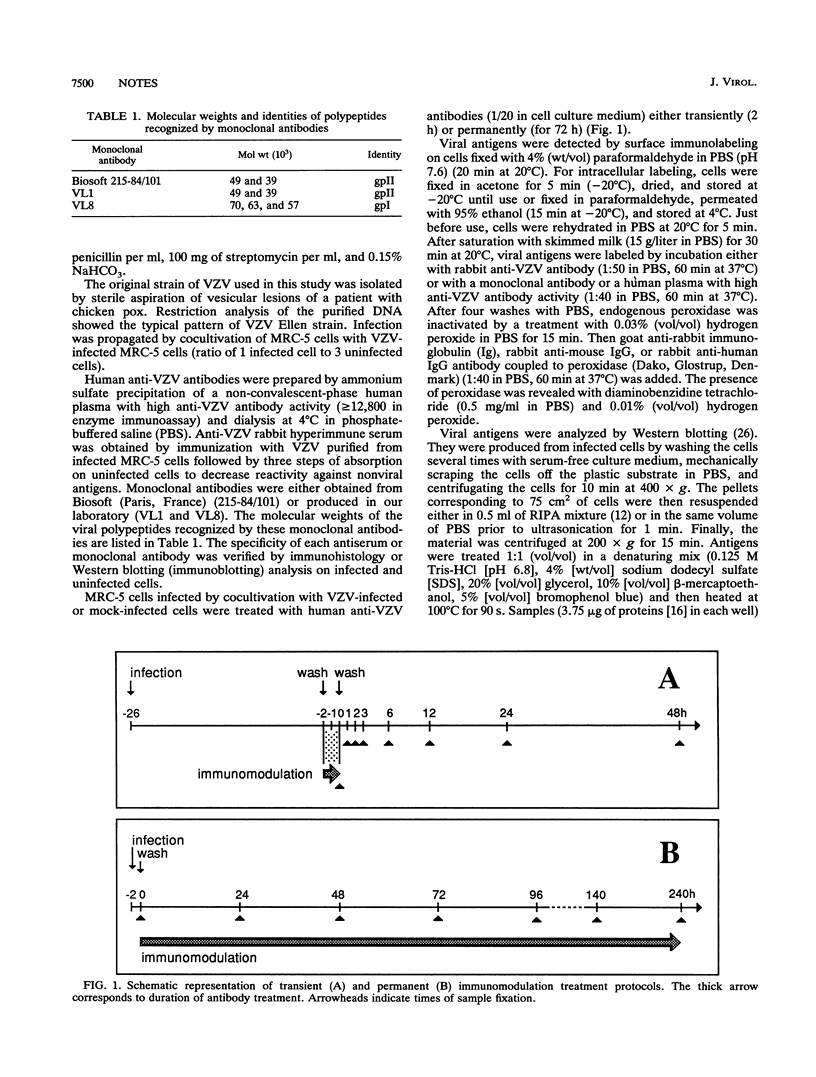
Abstract
Varicella-zoster virus (VZV) persists in human sensory ganglia. One of the hypotheses to explain the induction or the maintenance of VZV latency is that it could be promoted by the immune response itself. It is known that in the case of viruses which bud off the infected cell membrane, virus-specific antibodies can induce antigenic modulation, i.e., spatial redistribution of viral antigens and modulation of their synthesis. To determine whether antigenic modulation occurs during VZV infection in vitro and could possibly be involved in viral persistence, we have grown infected cells in the presence of anti-VZV antibodies either transiently or permanently. The distribution of immune complexes and viral proteins was then analyzed. In transient immunomodulation experiments, the distribution of one or more viral antigens was modified not only in the cytoplasmic membranes but also in the cytoplasm and nucleoplasm of infected cells. When infected cells were kept permanently in the presence of antibodies, the same pattern of redistribution of immune complexes was observed and the localization of internal viral glycoproteins was significantly modified. However, antibodies did not prevent the lytic effect of infection; they altered neither the infectious virus yield nor the Western immunoblot pattern of viral proteins, suggesting that immunomodulation is not the primary effector of viral persistence.
Full text
PDF

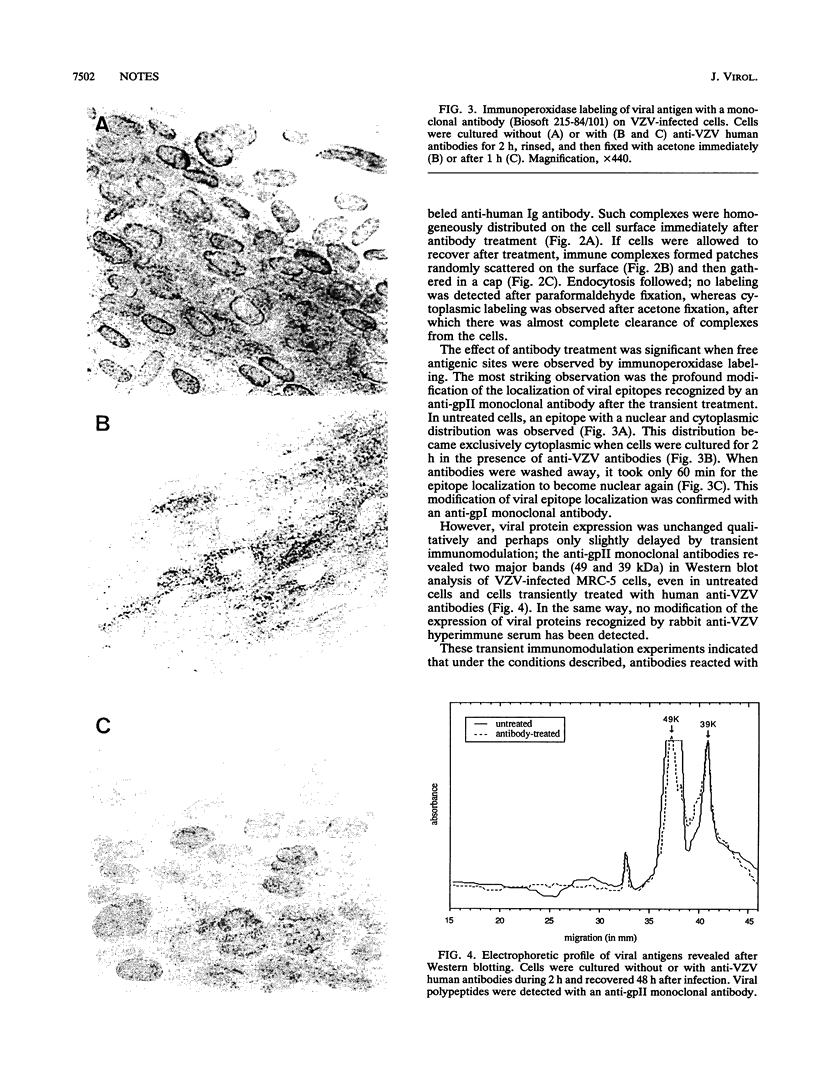
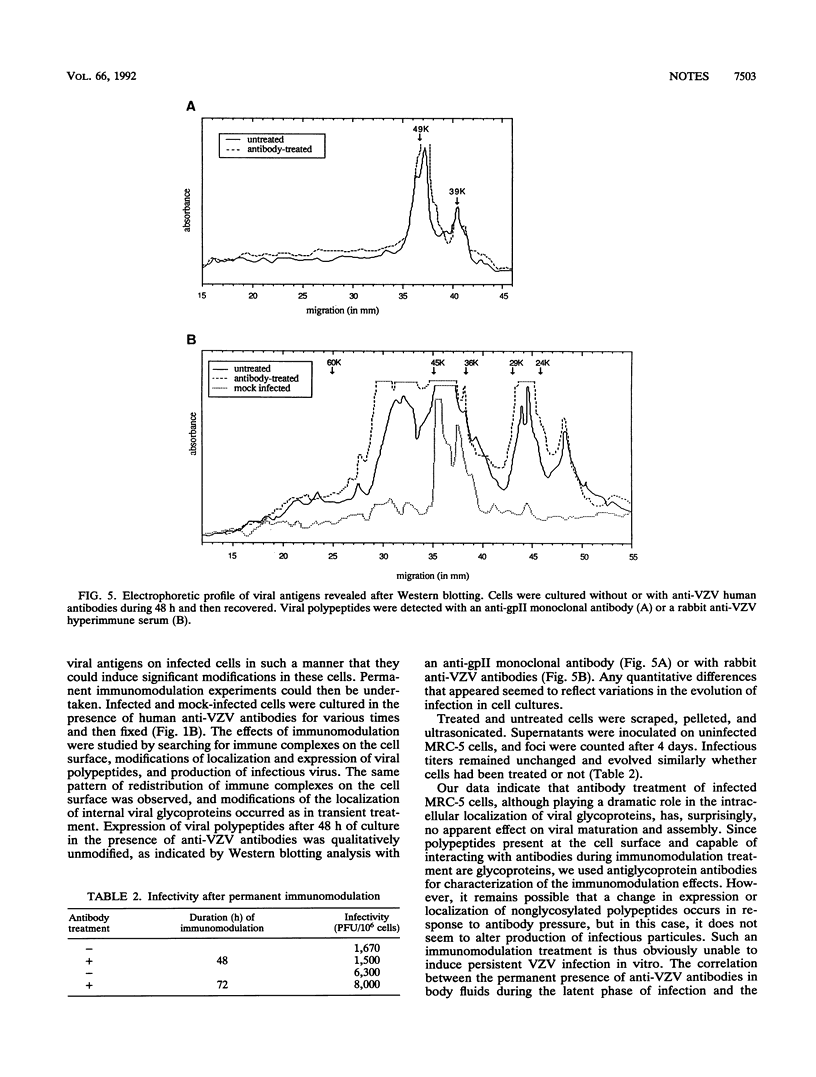

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Larsen S., Cohn A., Uttenthal A., Race R. E., Aasted B., Hansen M., Bloom M. E. Passive transfer of antiviral antibodies restricts replication of Aleutian mink disease parvovirus in vivo. J Virol. 1989 Jan;63(1):9–17. doi: 10.1128/jvi.63.1.9-17.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell P. A., Gershon A. A., Hughes W. T., Riley H. D., Jr, Smith J. Prevention of varicella in high risk children: a collaborative study. Pediatrics. 1972 Nov;50(5):718–722. [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Talbot P. J., Knobler R. L. Murine hepatitis virus-4 (strain JHM)-induced neurologic disease is modulated in vivo by monoclonal antibody. Virology. 1984 Jan 30;132(2):261–270. doi: 10.1016/0042-6822(84)90033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J., Rabson A. S., Yee C., Tralka T. S. Immunoglobulin binding to herpes virus-induced Fc receptors inhibits virus growth. Nature. 1977 Sep 15;269(5625):251–252. doi: 10.1038/269251a0. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Norrby E., Oldstone M. B. Antigenic modulation induced by monoclonal antibodies: antibodies to measles virus hemagglutinin alters expression of other viral polypeptides in infected cells. J Immunol. 1984 May;132(5):2618–2621. [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Alterations in expression of measles virus polypeptides by antibody: molecular events in antibody-induced antigenic modulation. J Immunol. 1980 Jul;125(1):78–85. [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979 Jun 7;279(5713):529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- Gould J. J., Almeida J. D. Antibody-modification of measles "in vitro" infection. J Med Virol. 1977;1(2):111–117. doi: 10.1002/jmv.1890010204. [DOI] [PubMed] [Google Scholar]
- HOPE-SIMPSON R. E. THE NATURE OF HERPES ZOSTER: A LONG-TERM STUDY AND A NEW HYPOTHESIS. Proc R Soc Med. 1965 Jan;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooghe-Peters E. L., Rentier B., Dubois-Dalcq M. Electron microscopic study of measles virus infection: unusual antibody-triggered redistribution of antigens on giant cells. J Virol. 1979 Feb;29(2):666–676. doi: 10.1128/jvi.29.2.666-676.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P. J., Kjellén L. Inhibition of herpes simplex virus growth caused by preparations of animal immunoglobulins is not dependent on Fc-Fc receptor interactions. Intervirology. 1988;29(6):334–338. doi: 10.1159/000150064. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Joseph B. S., Oldstone M. B. Antibody-induced capping of measles virus antigens on plasma membrane studied by electron microscopy. J Virol. 1975 May;15(5):1248–1255. doi: 10.1128/jvi.15.5.1248-1255.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert U. G., Schneider-Schaulies S., Baczko K., ter Meulen V. Antibody-induced restriction of viral gene expression in measles encephalitis in rats. J Virol. 1990 Feb;64(2):706–713. doi: 10.1128/jvi.64.2.706-713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor F. Structure and dynamics of the lymphocyte surface, in relation to differentiation, recognition and activation. Prog Allergy. 1977;23:1–153. [PubMed] [Google Scholar]
- Oldstone M. B., Fujinami R. S., Lampert P. W. Membrane and cytoplasmic changes in virus-infected cells induced by interactions of antiviral antibody with surface viral antigen. Prog Med Virol. 1980;26:45–93. [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A. Immunologic injury in measles virus infection. IV. Antigens modulation and abrogation oflymphocyte lysis of virus-infected cells. Clin Immunol Immunopathol. 1978 Jan;9(1):55–62. doi: 10.1016/0090-1229(78)90120-4. [DOI] [PubMed] [Google Scholar]
- Phillips E. R., Perdue J. F. The dynamics of antibody-induced redistribution of viral envelope antigens in the plasma membranes of avian tumour virus-infected chick embryo fibroblasts. J Cell Sci. 1976 May;20(3):459–477. doi: 10.1242/jcs.20.3.459. [DOI] [PubMed] [Google Scholar]
- Rammohan K. W., McFarland H. F., McFarlin D. E. Induction of subacute murine measles encephalitis by monoclonal antibody to virus haemagglutinin. Nature. 1981 Apr 16;290(5807):588–589. doi: 10.1038/290588a0. [DOI] [PubMed] [Google Scholar]
- Rammohan K. W., McFarland H. F., McFarlin D. E. Subacute sclerosing panencephalitis after passive immunization and natural measles infection: role of antibody in persistence of measles virus. Neurology. 1982 Apr;32(4):390–394. doi: 10.1212/wnl.32.4.390. [DOI] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. II. Effect of measles antibody on persistently infected HeLa sublines and recovery of a HeLa clonal line persistently infected with incomplete virus. J Bacteriol. 1966 Dec;92(6):1805–1811. doi: 10.1128/jb.92.6.1805-1811.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter G., Mannweiler K. Antibody-induced redistribution of virus antigens on the surface of influenza virus-infected cells. J Gen Virol. 1976 Nov;33(2):321–332. doi: 10.1099/0022-1317-33-2-321. [DOI] [PubMed] [Google Scholar]
- Stackpole C. W., Jacobson J. B., Lardis M. P. Antigenic modulation in vitro. I. Fate of thymus-leukemia (TL) antigen-antibody complexes following modulation of TL antigenicity from the surfaces of mouse leukemia cells and thymocytes. J Exp Med. 1974 Oct 1;140(4):939–953. doi: 10.1084/jem.140.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]