Abstract
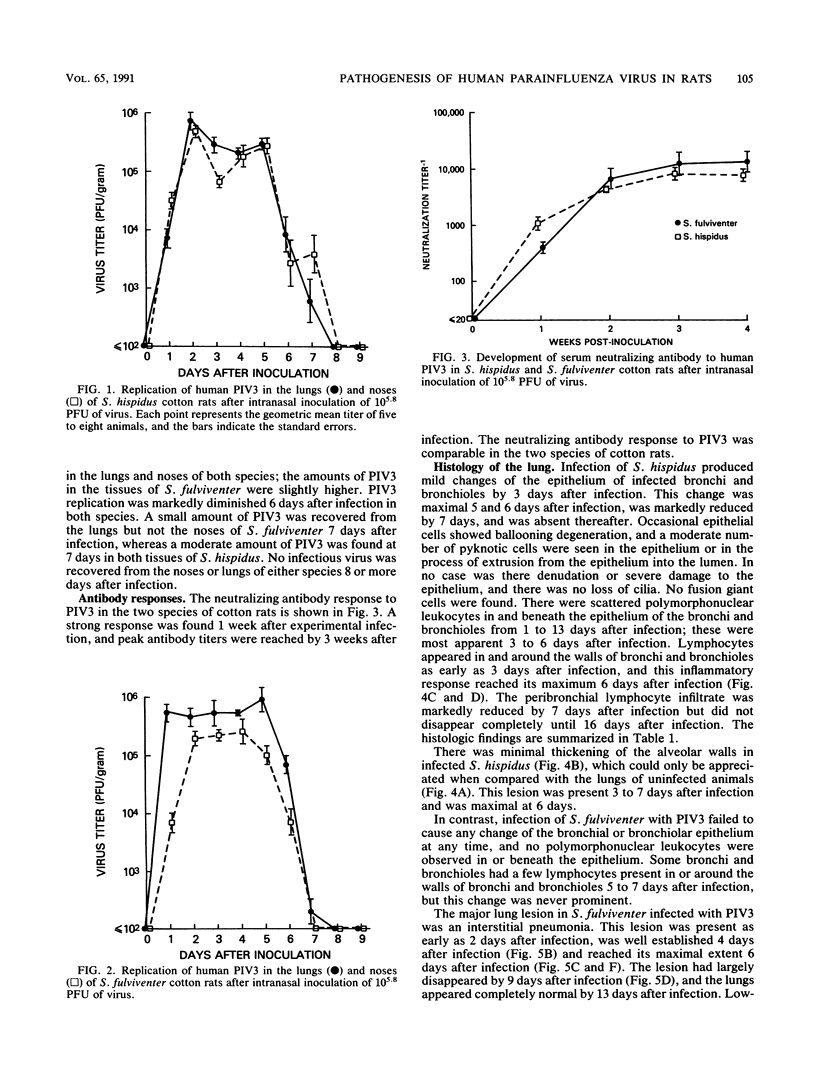
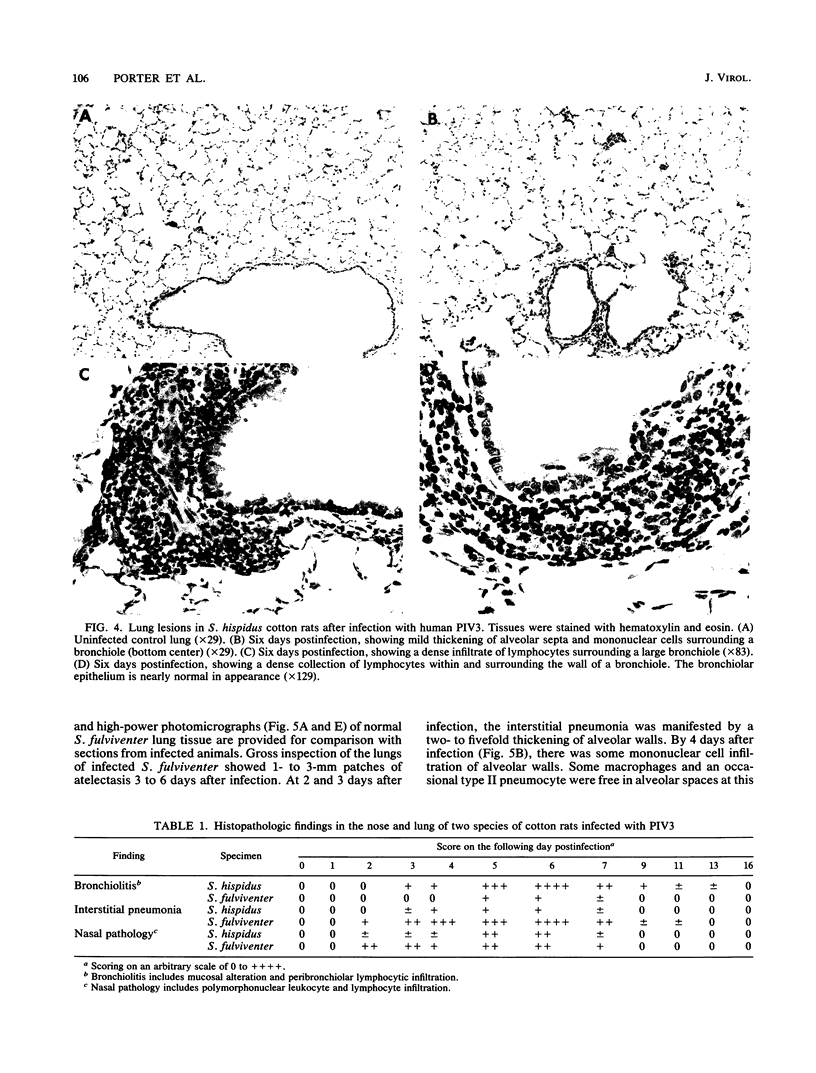
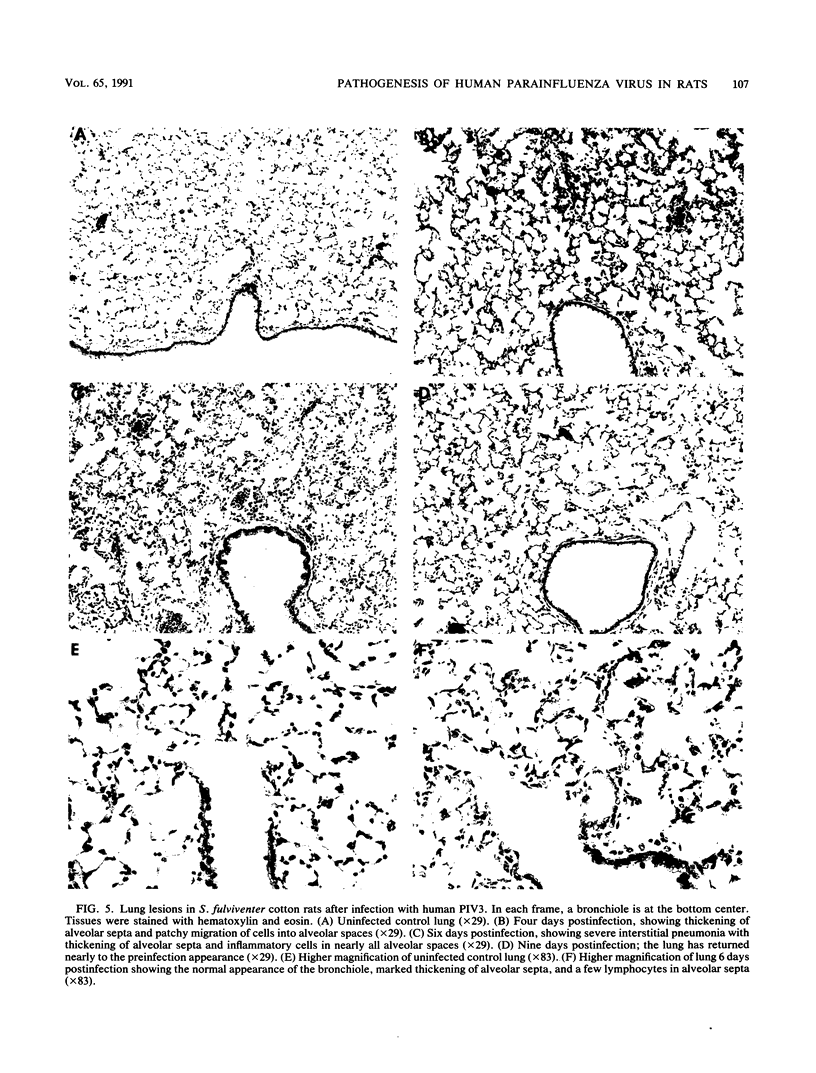
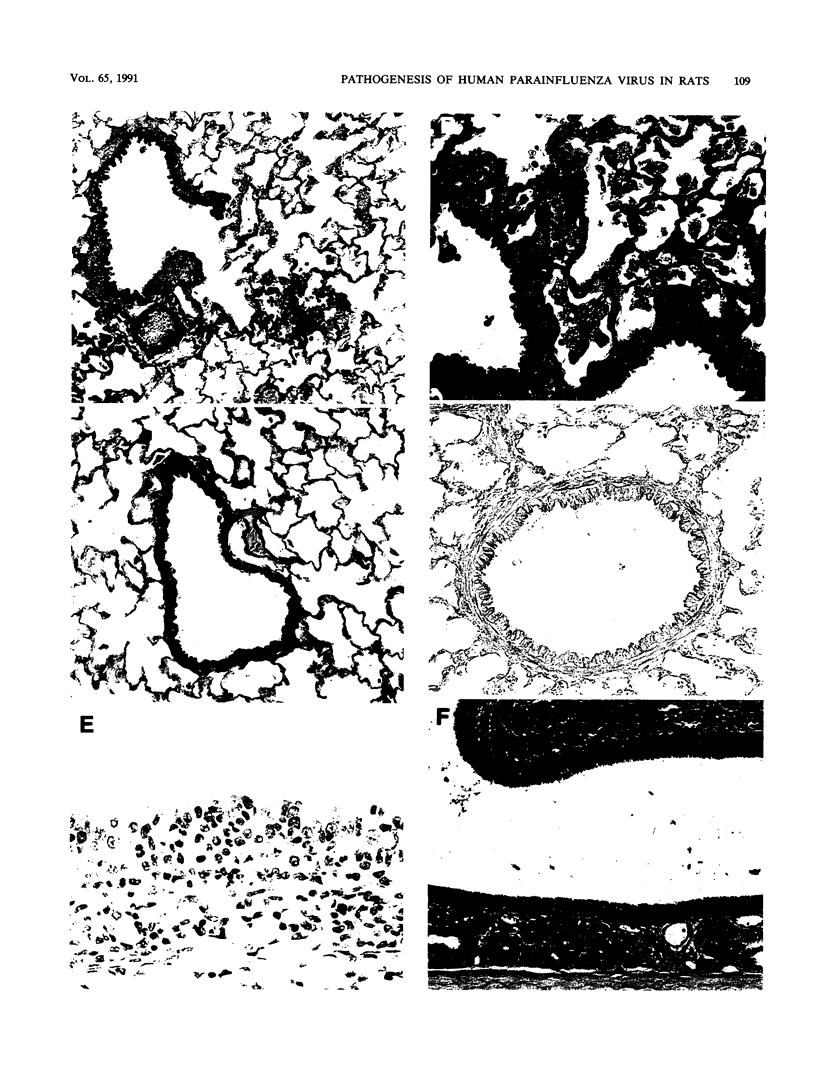
Human parainfluenza virus 3 replicates well in the noses and lungs of two species of cotton rats, Sigmodon hispidus and Sigmodon fulviventer. Peak viral titers of nearly 10(6) PFU/g are reached 2 days after infection in both tissues, are maintained through day 5, and are equivalent in the two species. Infectious virus is eliminated by day 8 after infection. Both species produce a strong neutralizing antibody response with titers of 1:10,000 4 weeks after infection. Viral replication in the nasal epithelium results in only minor histological changes, and viral antigen is found only in the apical portion of epithelial cells. Infection of S. hispidus causes a bronchiolitis with a peribronchiolar lymphoid cell infiltration that reaches a peak 6 days after infection, and there is only a minor component of interstitial pneumonia. In contrast, infection of S. fulviventer causes an interstitial pneumonia, and this lesion reaches its maximal extent by 6 days after infection. There is minimal peribronchiolar lymphoid cell infiltration in infected S. fulviventer. Lung lesions in both species of cotton rats are largely healed 9 days after infection, and the lungs are indistinguishable from those of uninfected controls 16 days after infection. These species of cotton rats offer separate models for the two major pulmonary manifestations of human parainfluenza virus 3 infection. The models may be useful for basic studies of the pathogenesis of this infection and for initial evaluation of candidate vaccines.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABINANTI F. R., BYRNE R. J., WATSON R. L., POELMA L. J., LUCAS F. R., HUEBNER R. J. Observations on infections of cattle with myxovirus para-influenza 3. Am J Hyg. 1960 Jan;71:52–58. doi: 10.1093/oxfordjournals.aje.a120089. [DOI] [PubMed] [Google Scholar]
- Aherne W., Bird T., Court S. D., Gardner P. S., McQuillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970 Feb;23(1):7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appell L. H., Kovatch R. M., Reddecliff J. M., Gerone P. J. Pathogenesis of Sendai virus infection in mice. Am J Vet Res. 1971 Nov;32(11):1835–1841. [PubMed] [Google Scholar]
- BANG B. G., BANG F. B. A comparative study of the vertebrate nasal chamber in relation to upper respiratory infections. Bull Johns Hopkins Hosp. 1959 Mar;104(3):107–149. [PubMed] [Google Scholar]
- BUTHALA D. A., SORET M. G. PARAINFLUENZA TYPE 3 VIRUS INFECTION IN HAMSTERS: VIROLOGIC, SEROLOGIC, AND PATHOLOGIC STUDIES. J Infect Dis. 1964 Jun;114:226–234. doi: 10.1093/infdis/114.3.226. [DOI] [PubMed] [Google Scholar]
- CRAIGHEAD J. E., COOK M. K., CHANOCK R. M. Infection of hamsters with para influenza 3 virus. Proc Soc Exp Biol Med. 1960 Jun;104:301–304. doi: 10.3181/00379727-104-25816. [DOI] [PubMed] [Google Scholar]
- Coelingh K. J., Winter C. C., Murphy B. R., Rice J. M., Kimball P. C., Olmsted R. A., Collins P. L. Conserved epitopes on the hemagglutinin-neuraminidase proteins of human and bovine parainfluenza type 3 viruses: nucleotide sequence analysis of variants selected with monoclonal antibodies. J Virol. 1986 Oct;60(1):90–96. doi: 10.1128/jvi.60.1.90-96.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookshanks F. K., Belshe R. B. Evaluation of cold-adapted and temperature-sensitive mutants of parainfluenza virus type 3 in weanling hamsters. J Med Virol. 1984;13(3):243–249. doi: 10.1002/jmv.1890130306. [DOI] [PubMed] [Google Scholar]
- Cutlip R. C., Lehmkuhl H. D. Experimentally induced parainfluenza type 3 virus infection in young lambs: pathologic response. Am J Vet Res. 1982 Dec;43(12):2101–2107. [PubMed] [Google Scholar]
- Delage G., Brochu P., Pelletier M., Jasmin G., Lapointe N. Giant-cell pneumonia caused by parainfluenza virus. J Pediatr. 1979 Mar;94(3):426–429. doi: 10.1016/s0022-3476(79)80591-0. [DOI] [PubMed] [Google Scholar]
- Downham M. A., Gardner P. S., McQuillin J., Ferris J. A. Role of respiratory viruses in childhood mortality. Br Med J. 1975 Feb 1;1(5952):235–239. doi: 10.1136/bmj.1.5952.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J. A., Jr, Warren R. W., Tucker J. A., Zeller J., Wilfert C. M. Disseminated parainfluenza infection in a child with severe combined immunodeficiency. Am J Dis Child. 1983 Dec;137(12):1172–1174. doi: 10.1001/archpedi.1983.02140380032010. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Fernald G. W. Effect of passive antibody on parainfluenza virus type 3 pneumonia in hamsters. Infect Immun. 1976 Jul;14(1):212–216. doi: 10.1128/iai.14.1.212-216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen W. P., Frank A. L., Taber L. H., Kasel J. A. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis. 1984 Dec;150(6):851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- Henderson F. W. Pulmonary cell-mediated cytotoxicity in hamsters with parainfluenza virus type 3 pneumonia. Am Rev Respir Dis. 1979 Jul;120(1):41–47. doi: 10.1164/arrd.1979.120.1.41. [DOI] [PubMed] [Google Scholar]
- Jarvis W. R., Middleton P. J., Gelfand E. W. Parainfluenza pneumonia in severe combined immunodeficiency disease. J Pediatr. 1979 Mar;94(3):423–425. doi: 10.1016/s0022-3476(79)80590-9. [DOI] [PubMed] [Google Scholar]
- MCLEAN K. H. The pathology of acute bronchiolitis; a study of its evolution. I. The exudative phase. Australas Ann Med. 1956 Nov;5(4):254–267. doi: 10.1111/imj.1956.5.4.254. [DOI] [PubMed] [Google Scholar]
- MCLEAN K. H. The pathology of acute bronchiolitis; a study of its evolution. II. The repair phase. Australas Ann Med. 1957 Feb;6(1):29–concl. doi: 10.1111/imj.1957.6.1.29. [DOI] [PubMed] [Google Scholar]
- MORISON J. E. Pneumonia and interstitial inflammation of the lung. Lancet. 1955 Nov 5;269(6897):941–944. doi: 10.1016/s0140-6736(55)92788-4. [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Dubovi E. J., Clyde W. A., Jr The cotton rat as an experimental model of human parainfluenza virus type 3 disease. Exp Lung Res. 1981 May;2(2):97–109. doi: 10.3109/01902148109052306. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Chanock R. M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985 Sep;55(3):517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Hemming V. G., Murphy B. R., Walsh E. E., Horswood R. L., Chanock R. M. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986 Mar;57(3):721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Horswood R. L., Camargo E., Chanock R. M. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978 Dec;93(3):771–791. [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Porter D. D. The pathogenesis of respiratory syncytial virus infection in infant ferrets. Am J Pathol. 1976 Feb;82(2):339–352. [PMC free article] [PubMed] [Google Scholar]
- Profeta M. L., Lief F. S., Plotkin S. A. Enzootic sendai infection in laboratory hamsters. Am J Epidemiol. 1969 Mar;89(3):316–324. doi: 10.1093/oxfordjournals.aje.a120944. [DOI] [PubMed] [Google Scholar]
- Ray R., Glaze B. J., Compans R. W. Role of individual glycoproteins of human parainfluenza virus type 3 in the induction of a protective immune response. J Virol. 1988 Mar;62(3):783–787. doi: 10.1128/jvi.62.3.783-787.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M. K., Murphy B. R., Prince G. A., Olmsted R. A., Collins P. L. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: contributions of the individual proteins to host immunity. J Virol. 1987 Nov;61(11):3416–3423. doi: 10.1128/jvi.61.11.3416-3423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffin S. C., Muck K. B., Young J. C., Lewin K., Porter D. D. Improvement of the glucose oxidase immunoenzyme technic. Use of a tetrazolium whose formazan is stable without heavey metal chelation. Am J Clin Pathol. 1979 May;71(5):492–496. doi: 10.1093/ajcp/71.5.492. [DOI] [PubMed] [Google Scholar]