Abstract
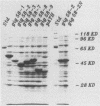
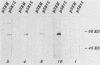
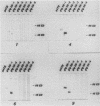
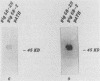
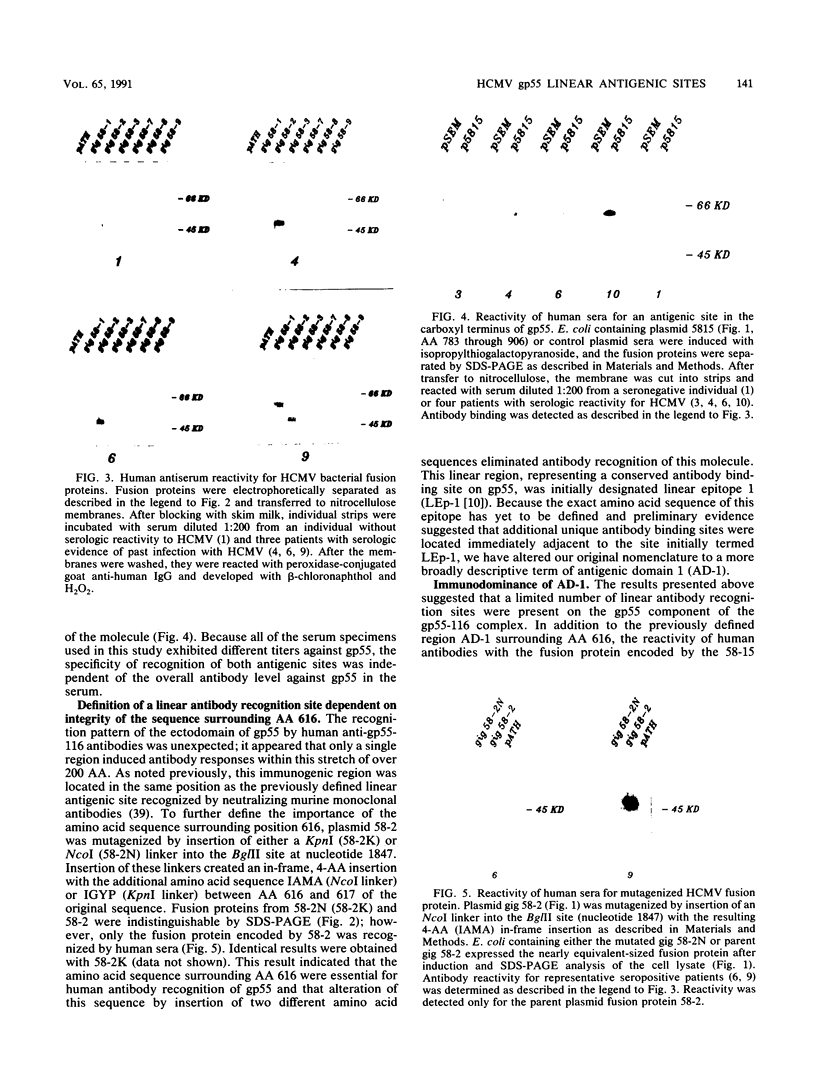
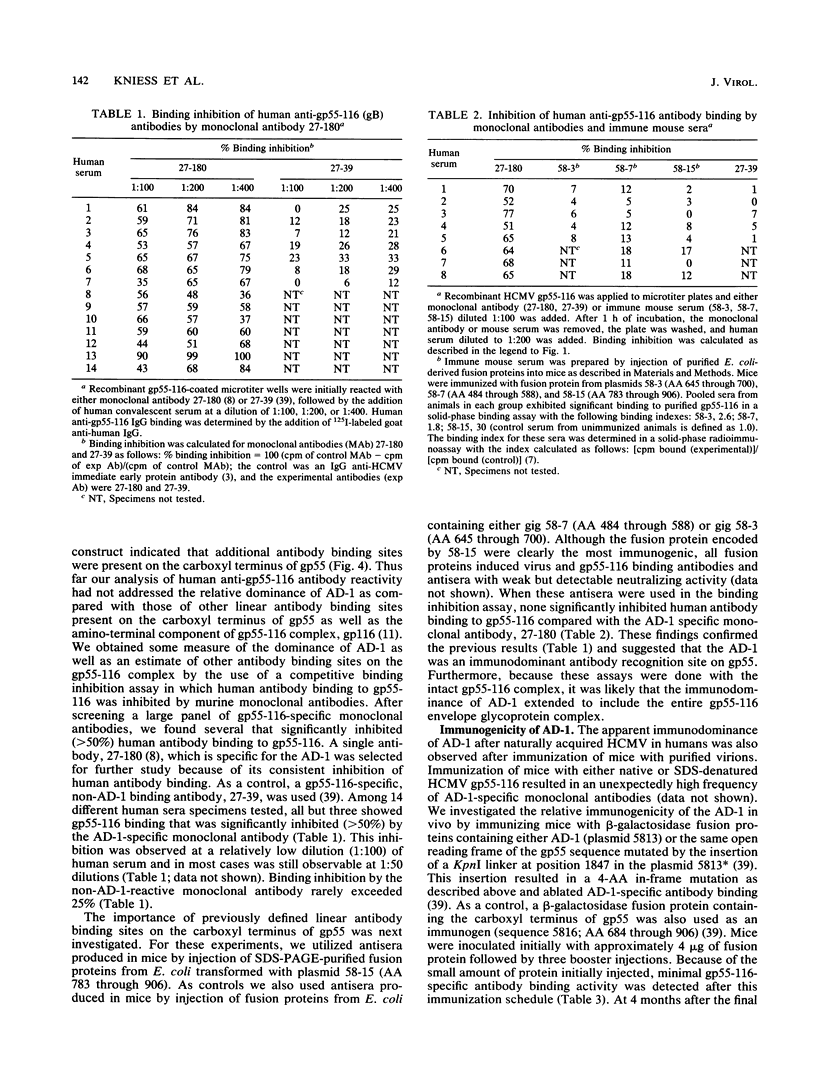
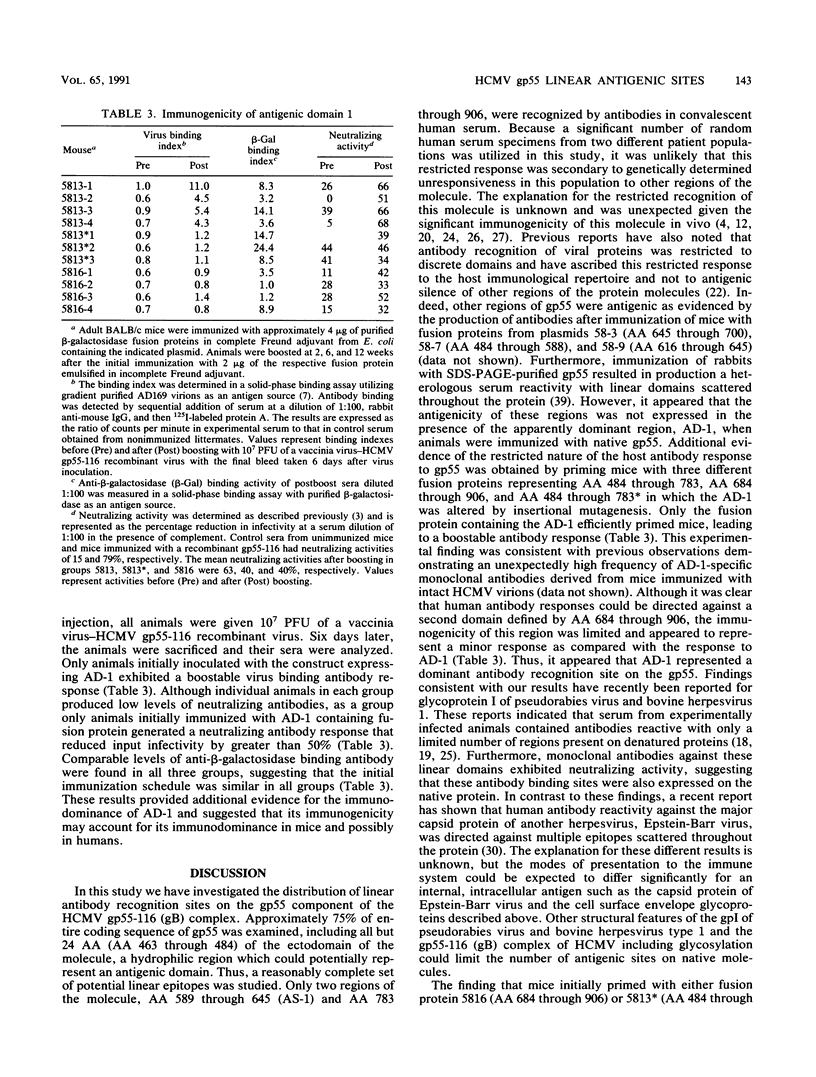
Human convalescent serum and bacterial fusion proteins constructed from overlapping open reading frames of the nucleotide sequence encoding the human cytomegalovirus gp55 component of the major envelope glycoprotein complex, gp55-116 (gB), were used to localize antigenic regions recognized by human antibodies. All donor serum analyzed contained antibody reactivity for an antigenic site(s) located between amino acids (AA) 589 and 645, a region containing a previously defined linear site recognized by neutralizing monoclonal antibodies (U. Utz, B. Britt, L. Vugler, and M. Mach, J. Virol. 63:1995-2001, 1989). Furthermore, in-frame insertion of two different synthetic oligonucleotides encoding four amino acids into the sequence at nucleotide 1847 (AA 616) eliminated antibody recognition of the fusion protein. A second antibody binding site was located within the carboxyl terminus of the protein (AA 703 through 906). A competitive binding inhibition assay in which monoclonal antibodies were used to inhibit human antibody reactivity with recombinant gp55-116 (gB) suggested that the majority of human anti-gp55-116 (gB) antibodies were directed against a single antigenic region located between AA 589 and 645. Furthermore, inoculation of mice with fusion proteins containing this antigenic site led to a boostable antibody response. These results indicated that the antigenic site(s) located between AA 589 and 645 was an immunodominant antibody recognition site on gp55 and likely the whole gp55-116 (gB) molecule. The enhanced immunogenicity of this region in vivo may account for its immunodominance.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford C. A., Hayes K., Britt W. Primary cytomegalovirus infection in pregnancy: comparison of antibody responses to virus-encoded proteins between women with and without intrauterine infection. J Infect Dis. 1988 Nov;158(5):917–924. doi: 10.1093/infdis/158.5.917. [DOI] [PubMed] [Google Scholar]
- Alford C. A., Stagno S., Pass R. F. Natural history of perinatal cytomegaloviral infection. Ciba Found Symp. 1979;(77):125–147. doi: 10.1002/9780470720608.ch9. [DOI] [PubMed] [Google Scholar]
- Andreoni M., Faircloth M., Vugler L., Britt W. J. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J Virol Methods. 1989 Feb;23(2):157–167. doi: 10.1016/0166-0934(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Ashley R., Benedetti J., Corey L. Humoral immune response to HSV-1 and HSV-2 viral proteins in patients with primary genital herpes. J Med Virol. 1985 Oct;17(2):153–166. doi: 10.1002/jmv.1890170208. [DOI] [PubMed] [Google Scholar]
- Banks T., Huo B., Kousoulas K., Spaete R., Pachl C., Pereira L. A major neutralizing domain maps within the carboxyl-terminal half of the cleaved cytomegalovirus B glycoprotein. J Gen Virol. 1989 Apr;70(Pt 4):979–985. doi: 10.1099/0022-1317-70-4-979. [DOI] [PubMed] [Google Scholar]
- Bittle J. L., Houghten R. A., Alexander H., Shinnick T. M., Sutcliffe J. G., Lerner R. A., Rowlands D. J., Brown F. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature. 1982 Jul 1;298(5869):30–33. doi: 10.1038/298030a0. [DOI] [PubMed] [Google Scholar]
- Britt W. J., Auger D. Human cytomegalovirus virion-associated protein with kinase activity. J Virol. 1986 Jul;59(1):185–188. doi: 10.1128/jvi.59.1.185-188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Auger D. Synthesis and processing of the envelope gp55-116 complex of human cytomegalovirus. J Virol. 1986 Apr;58(1):185–191. doi: 10.1128/jvi.58.1.185-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984 Jun;135(2):369–378. doi: 10.1016/0042-6822(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Britt W. J., Vugler L. G. Antiviral antibody responses in mothers and their newborn infants with clinical and subclinical congenital cytomegalovirus infections. J Infect Dis. 1990 Feb;161(2):214–219. doi: 10.1093/infdis/161.2.214. [DOI] [PubMed] [Google Scholar]
- Britt W. J., Vugler L. G. Processing of the gp55-116 envelope glycoprotein complex (gB) of human cytomegalovirus. J Virol. 1989 Jan;63(1):403–410. doi: 10.1128/jvi.63.1.403-410.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Vugler L., Butfiloski E. J., Stephens E. B. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J Virol. 1990 Mar;64(3):1079–1085. doi: 10.1128/jvi.64.3.1079-1085.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Vugler L., Stephens E. B. Induction of complement-dependent and -independent neutralizing antibodies by recombinant-derived human cytomegalovirus gp55-116 (gB). J Virol. 1988 Sep;62(9):3309–3318. doi: 10.1128/jvi.62.9.3309-3318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Jameson B. A., Wimmer E. Priming for and induction of anti-poliovirus neutralizing antibodies by synthetic peptides. Nature. 1983 Aug 25;304(5928):699–703. doi: 10.1038/304699a0. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D. R., Redmond M. J., Attah-Poku S. K., van Drunen Littel-van den Hurk S., Babiuk L. A., Zamb T. J. Mapping of 10 epitopes on bovine herpesvirus type 1 glycoproteins gI and gIII. Virology. 1990 May;176(1):145–157. doi: 10.1016/0042-6822(90)90239-n. [DOI] [PubMed] [Google Scholar]
- Fuchs W., Rziha H. J., Lukàcs N., Braunschweiger I., Visser N., Lütticken D., Schreurs C. S., Thiel H. J., Mettenleiter T. C. Pseudorabies virus glycoprotein gI: in vitro and in vivo analysis of immunorelevant epitopes. J Gen Virol. 1990 May;71(Pt 5):1141–1151. doi: 10.1099/0022-1317-71-5-1141. [DOI] [PubMed] [Google Scholar]
- Gibson W., Irmiere A. Selection of particles and proteins for use as human cytomegalovirus subunit vaccines. Birth Defects Orig Artic Ser. 1984;20(1):305–324. [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Grose C., Litwin V. Immunology of the varicella-zoster virus glycoproteins. J Infect Dis. 1988 May;157(5):877–881. doi: 10.1093/infdis/157.5.877. [DOI] [PubMed] [Google Scholar]
- Gönczöl E., Hudecz F., Ianacone J., Dietzschold B., Starr S., Plotkin S. A. Immune responses to isolated human cytomegalovirus envelope proteins. J Virol. 1986 May;58(2):661–664. doi: 10.1128/jvi.58.2.661-664.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K., Alford C., Britt W. Antibody response to virus-encoded proteins after cytomegalovirus mononucleosis. J Infect Dis. 1987 Oct;156(4):615–621. doi: 10.1093/infdis/156.4.615. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Meloen R. H., Gielkens A. L., Van Oirschot J. T. Epitope analysis of glycoprotein I of pseudorabies virus. J Gen Virol. 1990 Apr;71(Pt 4):881–887. doi: 10.1099/0022-1317-71-4-881. [DOI] [PubMed] [Google Scholar]
- Landini M. P., Mirolo G., Coppolecchia P., Re M. C., La Placa M. Serum antibodies to individual cytomegalovirus structural polypeptides in renal transplant recipients during viral infection. Microbiol Immunol. 1986;30(7):683–695. doi: 10.1111/j.1348-0421.1986.tb02994.x. [DOI] [PubMed] [Google Scholar]
- Landini M. P., Re M. C., Mirolo G., Baldassarri B., La Placa M. Human immune response to cytomegalovirus structural polypeptides studied by immunoblotting. J Med Virol. 1985 Dec;17(4):303–311. doi: 10.1002/jmv.1890170403. [DOI] [PubMed] [Google Scholar]
- Lussenhop N. O., Goertz R., Wabuke-Bunoti M., Gehrz R., Kari B. Epitope analysis of human cytomegalovirus glycoprotein complexes using murine monoclonal antibodies. Virology. 1988 Jun;164(2):362–372. doi: 10.1016/0042-6822(88)90549-1. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Robert-Guroff M., Rusche J., Koito A., Hattori T., Hoshino H., Javaherian K., Takatsuki K., Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988 Jun;62(6):2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp J. M., Meloen R. H. Epitope-mapping on the Epstein-Barr virus major capsid protein using systematic synthesis of overlapping oligopeptides. J Virol Methods. 1988 Sep;21(1-4):147–159. doi: 10.1016/0166-0934(88)90061-4. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Balfour H. H., Jr, Marker S. C., Fryd D. S., Howard R. J., Simmons R. L. Cytomegalovirus disease in renal allograft recipients: a prospective study of the clinical features, risk factors and impact on renal transplantation. Medicine (Baltimore) 1980 Jul;59(4):283–300. [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Saxena A., Scott P. I., Song G. J., Probert W. S., Britt W. J., Gibson W., Rasmussen L., Pachl C. Sequence requirements for proteolytic processing of glycoprotein B of human cytomegalovirus strain Towne. J Virol. 1990 Jun;64(6):2922–2931. doi: 10.1128/jvi.64.6.2922-2931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Thayer R. M., Probert W. S., Masiarz F. R., Chamberlain S. H., Rasmussen L., Merigan T. C., Pachl C. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology. 1988 Nov;167(1):207–225. doi: 10.1016/0042-6822(88)90071-2. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Lerner R. A. Antibodies that react with predetermined sites on proteins. Science. 1983 Feb 11;219(4585):660–666. doi: 10.1126/science.6186024. [DOI] [PubMed] [Google Scholar]
- Utz U., Britt W., Vugler L., Mach M. Identification of a neutralizing epitope on glycoprotein gp58 of human cytomegalovirus. J Virol. 1989 May;63(5):1995–2001. doi: 10.1128/jvi.63.5.1995-2001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. E., Vugler L. G., Britt W. J. Structural and immunological characterization of human cytomegalovirus gp55-116 (gB) expressed in insect cells. J Gen Virol. 1990 Apr;71(Pt 4):873–880. doi: 10.1099/0022-1317-71-4-873. [DOI] [PubMed] [Google Scholar]
- Winston D. J., Ho W. G., Lin C. H., Bartoni K., Budinger M. D., Gale R. P., Champlin R. E. Intravenous immune globulin for prevention of cytomegalovirus infection and interstitial pneumonia after bone marrow transplantation. Ann Intern Med. 1987 Jan;106(1):12–18. doi: 10.7326/0003-4819-106-1-12. [DOI] [PubMed] [Google Scholar]
- Yeager A. S., Grumet F. C., Hafleigh E. B., Arvin A. M., Bradley J. S., Prober C. G. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981 Feb;98(2):281–287. doi: 10.1016/s0022-3476(81)80662-2. [DOI] [PubMed] [Google Scholar]
- Zaia J. A., Forman S. J., Ting Y. P., Vanderwal-Urbina E., Blume K. G. Polypeptide-specific antibody response to human cytomegalovirus after infection in bone marrow transplant recipients. J Infect Dis. 1986 Apr;153(4):780–787. doi: 10.1093/infdis/153.4.780. [DOI] [PubMed] [Google Scholar]