Abstract
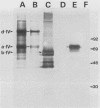
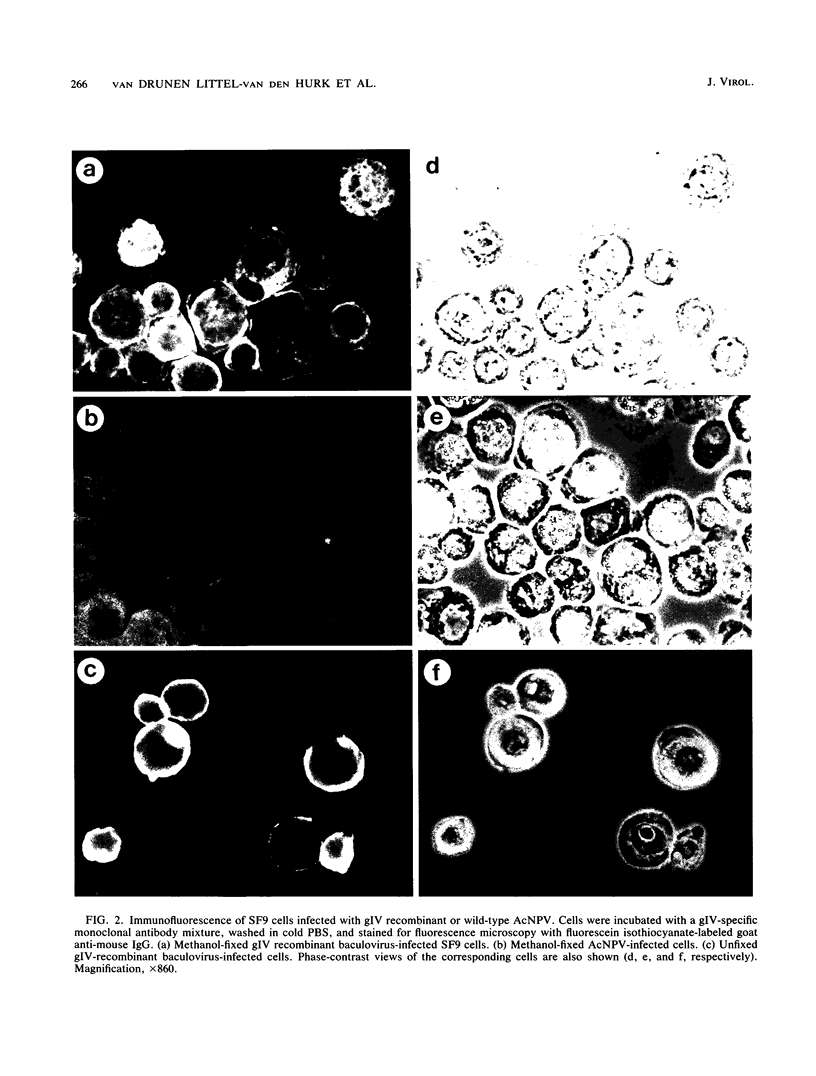
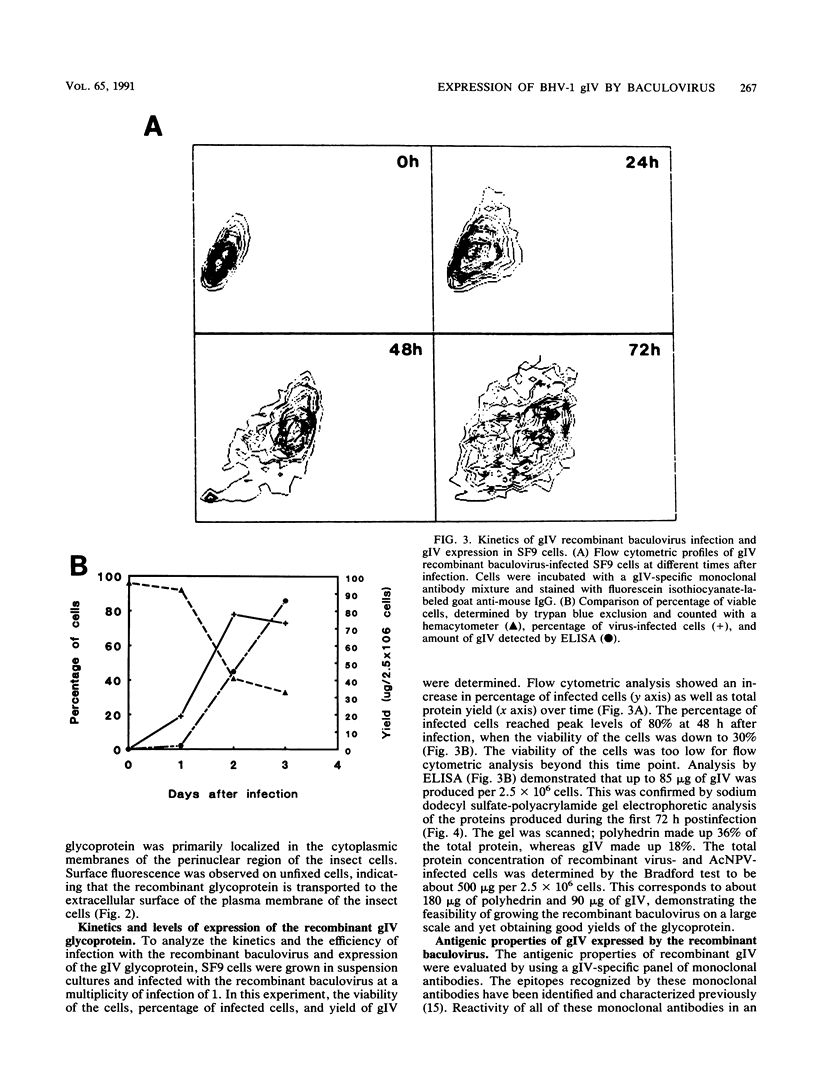
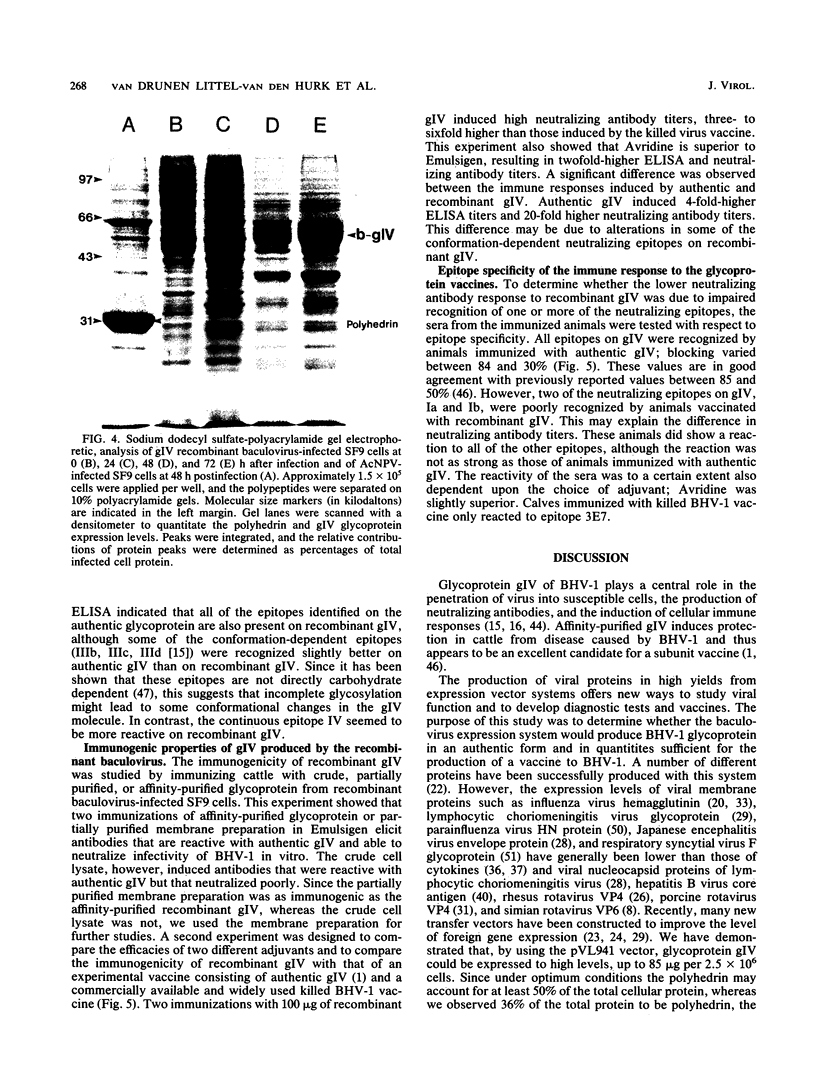
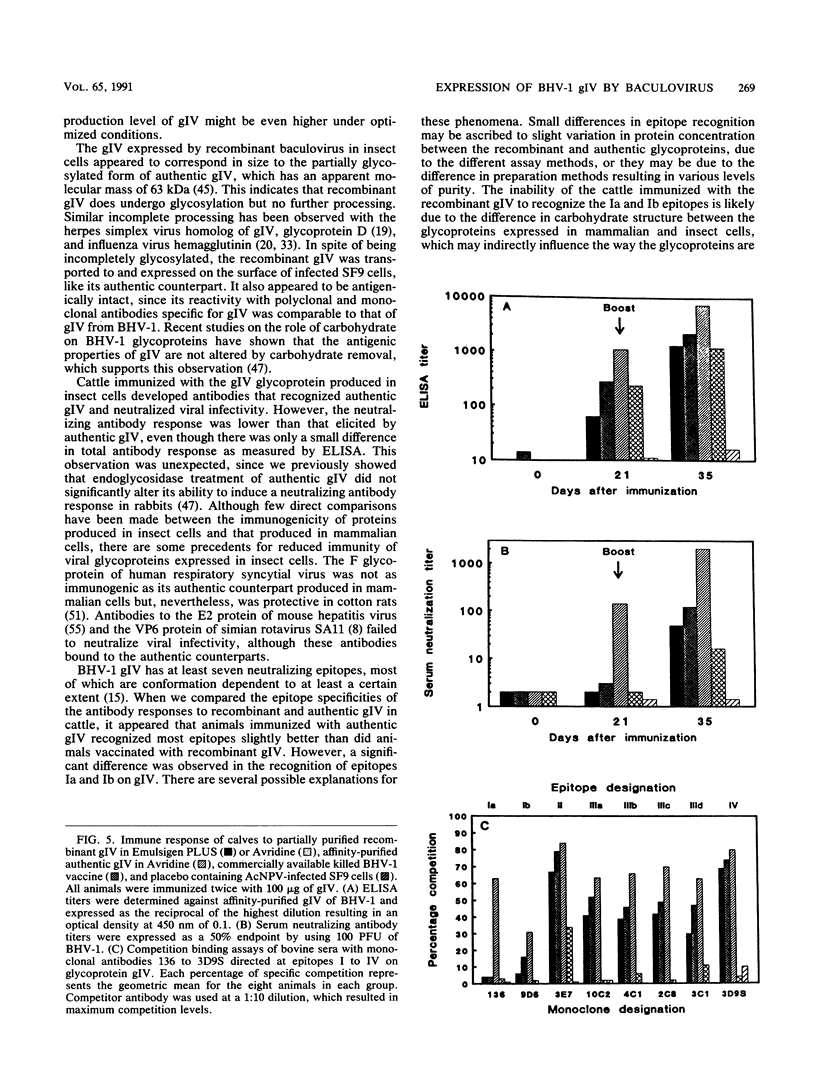
The gene encoding the gIV glycoprotein of bovine herpesvirus 1 has been inserted into the genome of Autographa californica baculovirus in lieu of the coding region of the A. californica baculovirus polyhedrin gene. Recombinant protein was identified by its reactivity with gIV-specific monoclonal antibodies and expressed at high levels (about 85 micrograms per 2.5 x 10(6) cells) in Spodoptera frugiperda (SF9) cells. The recombinant glycoprotein had an apparent molecular mass of 63 kDa, indicating that it was incompletely glycosylated. However, it was transported to and expressed on the cell surface of infected SF9 cells. Furthermore, reactivity with polyclonal and monoclonal antibodies specific for gIV suggested that most epitopes were functionally unaltered on the recombinant gIV. Immunization of cattle with recombinant gIV in crude, partially purified, or pure form resulted in the induction of neutralizing antibodies to BHV-1, which were reactive with authentic gIV. However, the neutralizing antibody titers were lower than those elicited by an equivalent amount of affinity-purified authentic gIV, which appeared to be mainly due to reduced recognition of one of the neutralizing antigenic domains of gIV, designated domain I. The potential use of this recombinant gIV glycoprotein as a vaccine to bovine herpesvirus 1 infection in cattle is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., L'Italien J., van Drunen Littel-van den Hurk S., Zamb T., Lawman J. P., Hughes G., Gifford G. A. Protection of cattle from bovine herpesvirus type I (BHV-1) infection by immunization with individual viral glycoproteins. Virology. 1987 Jul;159(1):57–66. doi: 10.1016/0042-6822(87)90347-3. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Wardley R. C., Rouse B. T. Defense mechanisms against bovine herpesvirus: relationship of virus-host cell events to susceptibility to antibody-complement cell lysis. Infect Immun. 1975 Nov;12(5):958–963. doi: 10.1128/iai.12.5.958-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Babiuk L. A. Viral-bacterial pneumonia in calves: effect of bovine herpesvirus-1 on immunologic functions. J Infect Dis. 1985 May;151(5):937–947. doi: 10.1093/infdis/151.5.937. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Arsenakis M., Farabegoli F., Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988 Jan;62(1):159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos M., Ohmann H. B., Hutchings D., Rapin N., Babiuk L. A., Lawman M. J. Role of interferon-gamma in inducing cytotoxicity of peripheral blood mononuclear leukocytes to bovine herpesvirus type 1 (BHV-1)-infected cells. Cell Immunol. 1989 Apr 15;120(1):259–269. doi: 10.1016/0008-8749(89)90193-7. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Isola V. J., Kuhns J., Berman P. W., Eisenberg R. J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing ("native" gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986 Oct;60(1):157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. K., Butcher A. C., Riegel C. A. Immune response to bovine herpes herpesvirus type 1 infections: virus-specific antibodies in sera from infected animals. J Clin Microbiol. 1985 Apr;21(4):546–552. doi: 10.1128/jcm.21.4.546-552.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Crawford S. E., Penaranda M. E., Petrie B. L., Burns J. W., Chan W. K., Ericson B., Smith G. E., Summers M. D. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987 May;61(5):1488–1494. doi: 10.1128/jvi.61.5.1488-1494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D. R., Zamb T., Parker M. D., van Drunen Littel-van den Hurk S., Babiuk L. A., Lawman M. J. Expression of bovine herpesvirus 1 glycoproteins gI and gIII in transfected murine cells. J Virol. 1988 Nov;62(11):4239–4248. doi: 10.1128/jvi.62.11.4239-4248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerichs G. N., Woods S. B., Lucas M. H., Sands J. J. Safety and efficacy of live and inactivated infectious bovine rhinotracheitis vaccines. Vet Rec. 1982 Aug 7;111(6):116–122. doi: 10.1136/vr.111.6.116. [DOI] [PubMed] [Google Scholar]
- Gerber J. D., Marron A. E., Kucera C. J. Local and systemic cellular and antibody immune responses of cattle to infectious bovine rhinotracheitis virus vaccines administered intranassally or intramuscularly. Am J Vet Res. 1978 May;39(5):753–760. [PubMed] [Google Scholar]
- Hackett C. J., Hurwitz J. L., Dietzschold B., Gerhard W. A synthetic decapeptide of influenza virus hemagglutinin elicits helper T cells with the same fine recognition specificities as occur in response to whole virus. J Immunol. 1985 Aug;135(2):1391–1394. [PubMed] [Google Scholar]
- Highlander S. L., Sutherland S. L., Gage P. J., Johnson D. C., Levine M., Glorioso J. C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987 Nov;61(11):3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G., Babiuk L. A., van Drunen Littel-van den Hurk S. Functional and topographical analyses of epitopes on bovine herpesvirus type 1 glycoprotein IV. Arch Virol. 1988;103(1-2):47–60. doi: 10.1007/BF01319808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings D. L., van Drunen Littel-van den Hurk S., Babiuk L. A. Lymphocyte proliferative responses to separated bovine herpesvirus 1 proteins in immune cattle. J Virol. 1990 Oct;64(10):5114–5122. doi: 10.1128/jvi.64.10.5114-5122.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jericho K. W., Babiuk L. A. The effect of dose, route and virulence of bovine herpesvirus 1 vaccine on experimental respiratory disease in cattle. Can J Comp Med. 1983 Apr;47(2):133–139. [PMC free article] [PubMed] [Google Scholar]
- Kimura-Kuroda J., Yasui K. Topographical analysis of antigenic determinants on envelope glycoprotein V3 (E) of Japanese encephalitis virus, using monoclonal antibodies. J Virol. 1983 Jan;45(1):124–132. doi: 10.1128/jvi.45.1.124-132.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S., Blacklaws B. A., Overton H. A., Bishop D. H., Nash A. A. Expression of glycoprotein D of herpes simplex virus type 1 in a recombinant baculovirus: protective responses and T cell recognition of the recombinant-infected cell extracts. J Gen Virol. 1989 Jul;70(Pt 7):1805–1814. doi: 10.1099/0022-1317-70-7-1805. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Hauser C., Rott R., Klenk H. D., Doerfler W. Expression of the influenza virus haemagglutinin in insect cells by a baculovirus vector. EMBO J. 1986 Jun;5(6):1359–1365. doi: 10.1002/j.1460-2075.1986.tb04367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawman M. J., Courtney R. J., Eberle R., Schaffer P. A., O'Hara M. K., Rouse B. T. Cell-mediated immunity to herpes simplex virus: specificity of cytotoxic T cells. Infect Immun. 1980 Nov;30(2):451–461. doi: 10.1128/iai.30.2.451-461.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. Signals important for high-level expression of foreign genes in Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1988 Nov;167(1):56–71. doi: 10.1016/0042-6822(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Macfarlan R. I., Dietzschold B., Wiktor T. J., Kiel M., Houghten R., Lerner R. A., Sutcliffe J. G., Koprowski H. T cell responses to cleaved rabies virus glycoprotein and to synthetic peptides. J Immunol. 1984 Nov;133(5):2748–2752. [PubMed] [Google Scholar]
- Mackow E. R., Barnett J. W., Chan H., Greenberg H. B. The rhesus rotavirus outer capsid protein VP4 functions as a hemagglutinin and is antigenically conserved when expressed by a baculovirus recombinant. J Virol. 1989 Apr;63(4):1661–1668. doi: 10.1128/jvi.63.4.1661-1668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura Y., Miyamoto M., Sato T., Morita C., Yasui K. Characterization of Japanese encephalitis virus envelope protein expressed by recombinant baculoviruses. Virology. 1989 Dec;173(2):674–682. doi: 10.1016/0042-6822(89)90580-1. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Overton H. A., Bishop D. H. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987 May;68(Pt 5):1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Mitchell D. An outbreak of abortion in a dairy herd following inoculation with an intramuscular infectious bovine rhinotracheitis virus vaccine. Can Vet J. 1974 May;15(5):148–151. [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K., Fukuhara N., Liprandi F., Green K., Kapikian A. Z., Chanock R. M., Gorziglia M. VP4 protein of porcine rotavirus strain OSU expressed by a baculovirus recombinant induces neutralizing antibodies. Virology. 1989 Dec;173(2):631–637. doi: 10.1016/0042-6822(89)90575-8. [DOI] [PubMed] [Google Scholar]
- Pastoret P. P., Babiuk L. A., Misra V., Griebel P. Reactivation of temperature-sensitive and non-temperature-sensitive infectious bovine rhinotracheitis vaccine virus with dexamethasone. Infect Immun. 1980 Aug;29(2):483–488. doi: 10.1128/iai.29.2.483-488.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possee R. D. Cell-surface expression of influenza virus haemagglutinin in insect cells using a baculovirus vector. Virus Res. 1986 Jul;5(1):43–59. doi: 10.1016/0168-1702(86)90064-x. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host defense mechanisms against infectious bovine rhinotracheitis virus. II. Inhibition of viral plaque formation by immune peripheral blood lymphocytes. Cell Immunol. 1975 May;17(1):43–56. doi: 10.1016/s0008-8749(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. The direct antiviral cytotoxicity by bovine lymphocytes is not restricted by genetic incompatibility of lymphocytes and target cells. J Immunol. 1977 Feb;118(2):618–624. [PubMed] [Google Scholar]
- Smith G. E., Ju G., Ericson B. L., Moschera J., Lahm H. W., Chizzonite R., Summers M. D. Modification and secretion of human interleukin 2 produced in insect cells by a baculovirus expression vector. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8404–8408. doi: 10.1073/pnas.82.24.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D., Fraser M. J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1983 Dec;3(12):2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Angelo C., Smith G. E., Summers M. D., Krug R. M. Two of the three influenza viral polymerase proteins expressed by using baculovirus vectors form a complex in insect cells. J Virol. 1987 Feb;61(2):361–365. doi: 10.1128/jvi.61.2.361-365.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K., Ireland D., Bishop D. H. Co-expression of the hepatitis B surface and core antigens using baculovirus multiple expression vectors. J Gen Virol. 1988 Nov;69(Pt 11):2763–2777. doi: 10.1099/0022-1317-69-11-2763. [DOI] [PubMed] [Google Scholar]
- Tikoo S. K., Fitzpatrick D. R., Babiuk L. A., Zamb T. J. Molecular cloning, sequencing, and expression of functional bovine herpesvirus 1 glycoprotein gIV in transfected bovine cells. J Virol. 1990 Oct;64(10):5132–5142. doi: 10.1128/jvi.64.10.5132-5142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa T., Ferguson M., Minor P. D., Cooper J., Sullivan M., Almond J. W., Bishop D. H. Synthesis of immunogenic, but non-infectious, poliovirus particles in insect cells by a baculovirus expression vector. J Gen Virol. 1989 Jun;70(Pt 6):1453–1463. doi: 10.1099/0022-1317-70-6-1453. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Brideau R. J., Thomsen D. R. Immunization of cotton rats with the human respiratory syncytial virus F glycoprotein produced using a baculovirus vector. J Infect Dis. 1989 Feb;159(2):255–264. doi: 10.1093/infdis/159.2.255. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Gariépy J., Schoolnik G. K., McConnell H. M. T-cell activation by peptide antigen: effect of peptide sequence and method of antigen presentation. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5480–5484. doi: 10.1073/pnas.82.16.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetstone C. A., Wheeler J. G., Reed D. E. Investigation of possible vaccine-induced epizootics of infectious bovine rhinotracheitis, using restriction endonuclease analysis of viral DNA. Am J Vet Res. 1986 Aug;47(8):1789–1795. [PubMed] [Google Scholar]
- Yates W. D. A review of infectious bovine rhinotracheitis, shipping fever pneumonia and viral-bacterial synergism in respiratory disease of cattle. Can J Comp Med. 1982 Jul;46(3):225–263. [PMC free article] [PubMed] [Google Scholar]
- Yoden S., Kikuchi T., Siddell S. G., Taguchi F. Expression of the peplomer glycoprotein of murine coronavirus JHM using a baculovirus vector. Virology. 1989 Dec;173(2):615–623. doi: 10.1016/0042-6822(89)90573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Babiuk L. A. Antigenic and immunogenic characteristics of bovine herpesvirus type-1 glycoproteins GVP 3/9 and GVP 6/11a/16, purified by immunoadsorbent chromatography. Virology. 1985 Jul 15;144(1):204–215. doi: 10.1016/0042-6822(85)90318-6. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Babiuk L. A. Polypeptide specificity of the antibody response after primary and recurrent infection with bovine herpesvirus 1. J Clin Microbiol. 1986 Feb;23(2):274–282. doi: 10.1128/jcm.23.2.274-282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Babiuk L. A. Synthesis and processing of bovine herpesvirus 1 glycoproteins. J Virol. 1986 Aug;59(2):401–410. doi: 10.1128/jvi.59.2.401-410.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Gifford G. A., Babiuk L. A. Epitope specificity of the protective immune response induced by individual bovine herpesvirus-1 glycoproteins. Vaccine. 1990 Aug;8(4):358–368. doi: 10.1016/0264-410x(90)90095-4. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Hughes G., Babiuk L. A. The role of carbohydrate in the antigenic and immunogenic structure of bovine herpesvirus type 1 glycoproteins gI and gIV. J Gen Virol. 1990 Sep;71(Pt 9):2053–2063. doi: 10.1099/0022-1317-71-9-2053. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., van den Hurk J. V., Babiuk L. A. Topographical analysis of bovine herpesvirus type-1 glycoproteins: use of monoclonal antibodies to identify and characterize functional epitopes. Virology. 1985 Jul 15;144(1):216–227. doi: 10.1016/0042-6822(85)90319-8. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., van den Hurk J. V., Gilchrist J. E., Misra V., Babiuk L. A. Interactions of monoclonal antibodies and bovine herpesvirus type 1 (BHV-1) glycoproteins: characterization of their biochemical and immunological properties. Virology. 1984 Jun;135(2):466–479. doi: 10.1016/0042-6822(84)90201-0. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Murphy B. R., Collins P. L., Lebacq-Verheyden A. M., Battey J. F. Expression of biologically active and antigenically authentic parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein by a recombinant baculovirus. Virology. 1987 Oct;160(2):465–472. doi: 10.1016/0042-6822(87)90018-3. [DOI] [PubMed] [Google Scholar]