Abstract
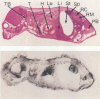
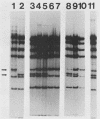
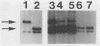
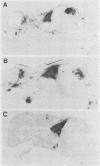
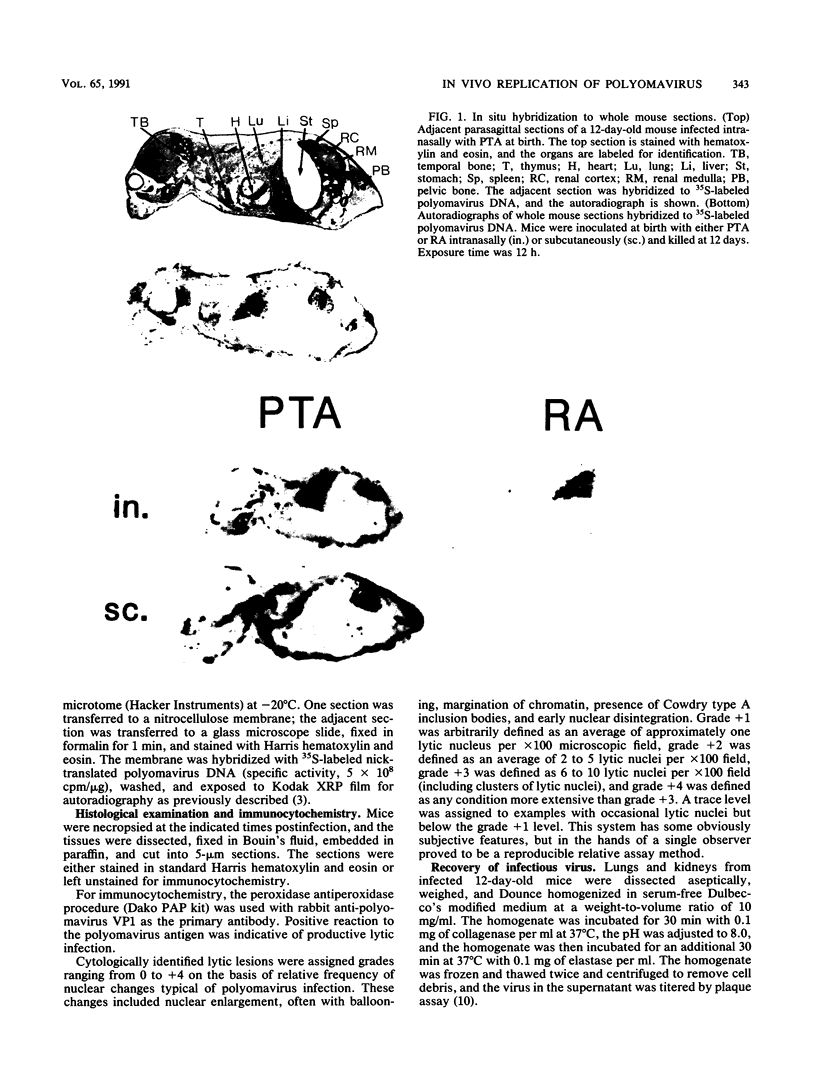
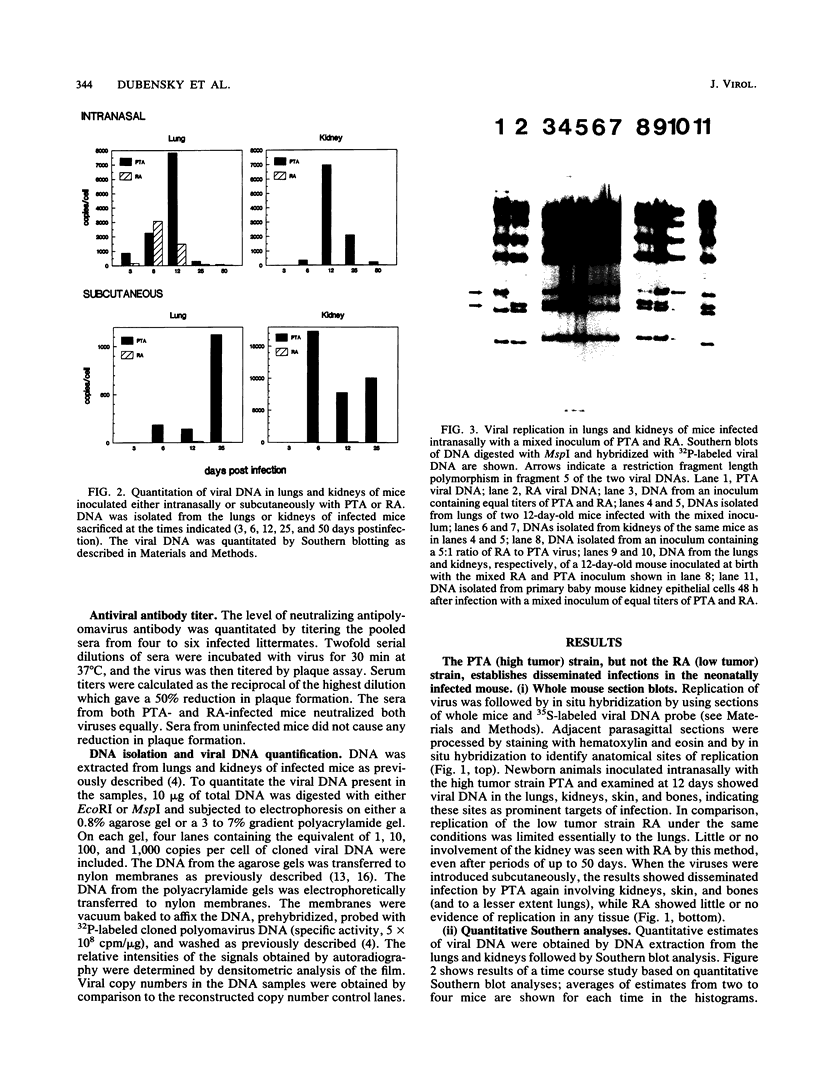
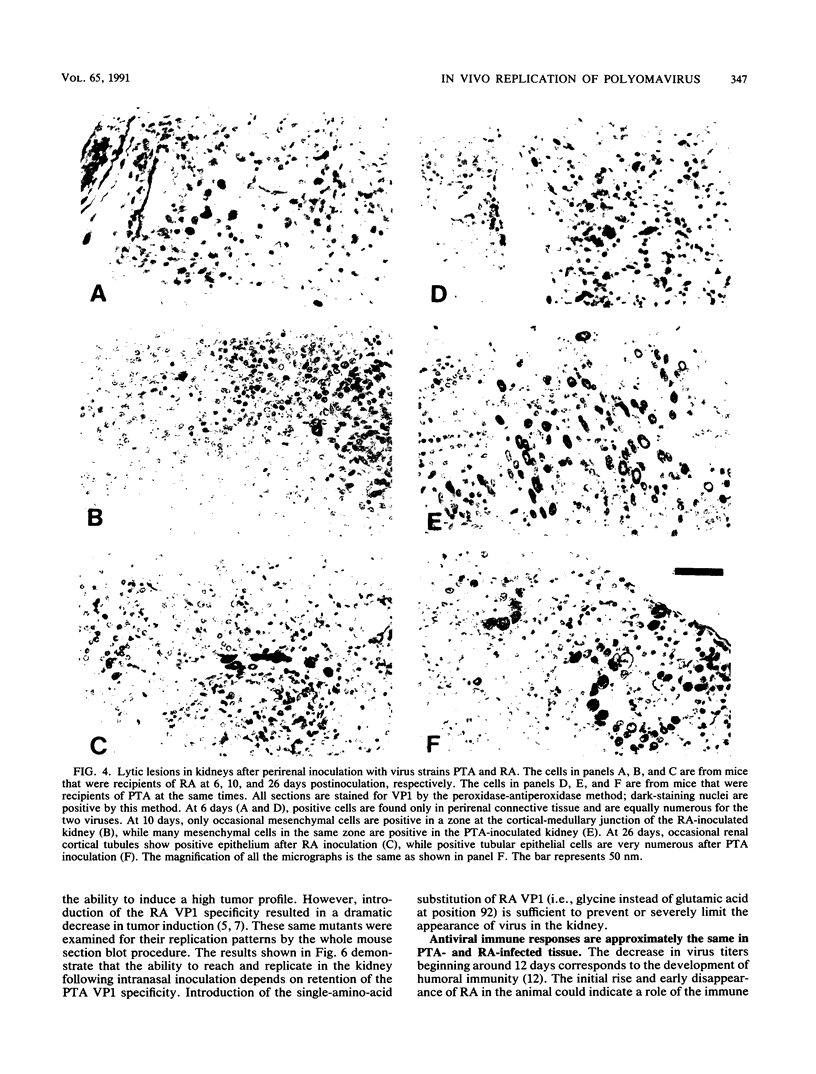
Patterns of polyomavirus replication and spread have been studied following inoculation of virus into newborn mice. Levels of virus replication in different tissues were followed in situ by using whole mouse section blots and immunoperoxidase staining for the major capsid protein VP1, as well as by tissue extraction and direct quantitation of viral DNA and infectious virus. Patterns of replication and spread were compared between the "high tumor" strain (inducing a high incidence of tumors) PTA and and the "low tumor" strain (inducing a low incidence of tumors) RA, following different routes of inoculation. The ability to induce a high tumor profile correlated with the ability to establish disseminated productive infection, with the kidney as a major site of amplification. Furthermore, results with PTA-RA recombinant viruses and site-directed mutants showed that the VP1 specificity of PTA, demonstrated earlier to be a critical determinant for induction of a high tumor profile (R. Freund, A. Calderone, C. J. Dawe, and T. L. Benjamin, J. Virol. 65:335-341, 1991), is also critical for amplification in the kidney and for establishment of disseminated infections.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawe C. J., Freund R., Barncastle J. P., Dubensky T. W., Mandel G., Benjamin T. L. Necrotizing arterial lesions in mice-bearing tumors induced by polyoma virus. J Exp Pathol. 1987 Spring;3(2):177–201. [PubMed] [Google Scholar]
- Dawe C. J., Freund R., Mandel G., Ballmer-Hofer K., Talmage D. A., Benjamin T. L. Variations in polyoma virus genotype in relation to tumor induction in mice. Characterization of wild type strains with widely differing tumor profiles. Am J Pathol. 1987 May;127(2):243–261. [PMC free article] [PubMed] [Google Scholar]
- Dubensky T. W., Murphy F. A., Villarreal L. P. Detection of DNA and RNA virus genomes in organ systems of whole mice: patterns of mouse organ infection by polyomavirus. J Virol. 1984 Jun;50(3):779–783. doi: 10.1128/jvi.50.3.779-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubensky T. W., Villarreal L. P. The primary site of replication alters the eventual site of persistent infection by polyomavirus in mice. J Virol. 1984 May;50(2):541–546. doi: 10.1128/jvi.50.2.541-546.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund R., Calderone A., Dawe C. J., Benjamin T. L. Polyomavirus tumor induction in mice: effects of polymorphisms of VP1 and large T antigen. J Virol. 1991 Jan;65(1):335–341. doi: 10.1128/jvi.65.1.335-341.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund R., Dawe C. J., Benjamin T. L. Duplication of noncoding sequences in polyomavirus specifically augments the development of thymic tumors in mice. J Virol. 1988 Oct;62(10):3896–3899. doi: 10.1128/jvi.62.10.3896-3899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund R., Dawe C. J., Benjamin T. L. The middle T proteins of high and low tumor strains of polyomavirus function equivalently in tumor induction. Virology. 1988 Dec;167(2):657–659. [PubMed] [Google Scholar]
- Freund R., Garcea R. L., Sahli R., Benjamin T. L. A single-amino-acid substitution in polyomavirus VP1 correlates with plaque size and hemagglutination behavior. J Virol. 1991 Jan;65(1):350–355. doi: 10.1128/jvi.65.1.350-355.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund R., Mandel G., Carmichael G. G., Barncastle J. P., Dawe C. J., Benjamin T. L. Polyomavirus tumor induction in mice: influences of viral coding and noncoding sequences on tumor profiles. J Virol. 1987 Jul;61(7):2232–2239. doi: 10.1128/jvi.61.7.2232-2239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman E., Benjamin T. L. Analysis of host range of nontransforming polyoma virus mutants. Virology. 1975 Aug;66(2):372–384. doi: 10.1016/0042-6822(75)90210-x. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ESTES J. D., HUEBNER R. J. Studies of mouse polyoma virus infection. 1. Procedures for quantitation and detection of virus. J Exp Med. 1959 Apr 1;109(4):379–391. doi: 10.1084/jem.109.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Talmage D. A., Freund R., Young A. T., Dahl J., Dawe C. J., Benjamin T. L. Phosphorylation of middle T by pp60c-src: a switch for binding of phosphatidylinositol 3-kinase and optimal tumorigenesis. Cell. 1989 Oct 6;59(1):55–65. doi: 10.1016/0092-8674(89)90869-6. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]