Abstract
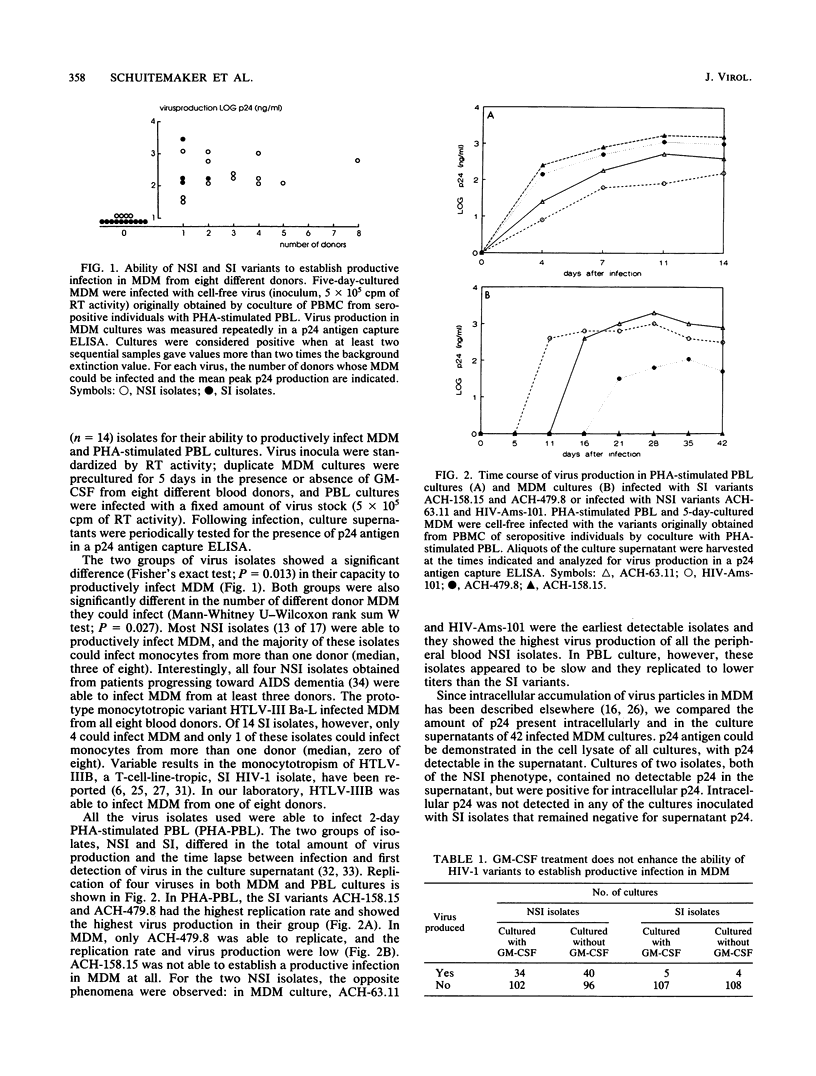
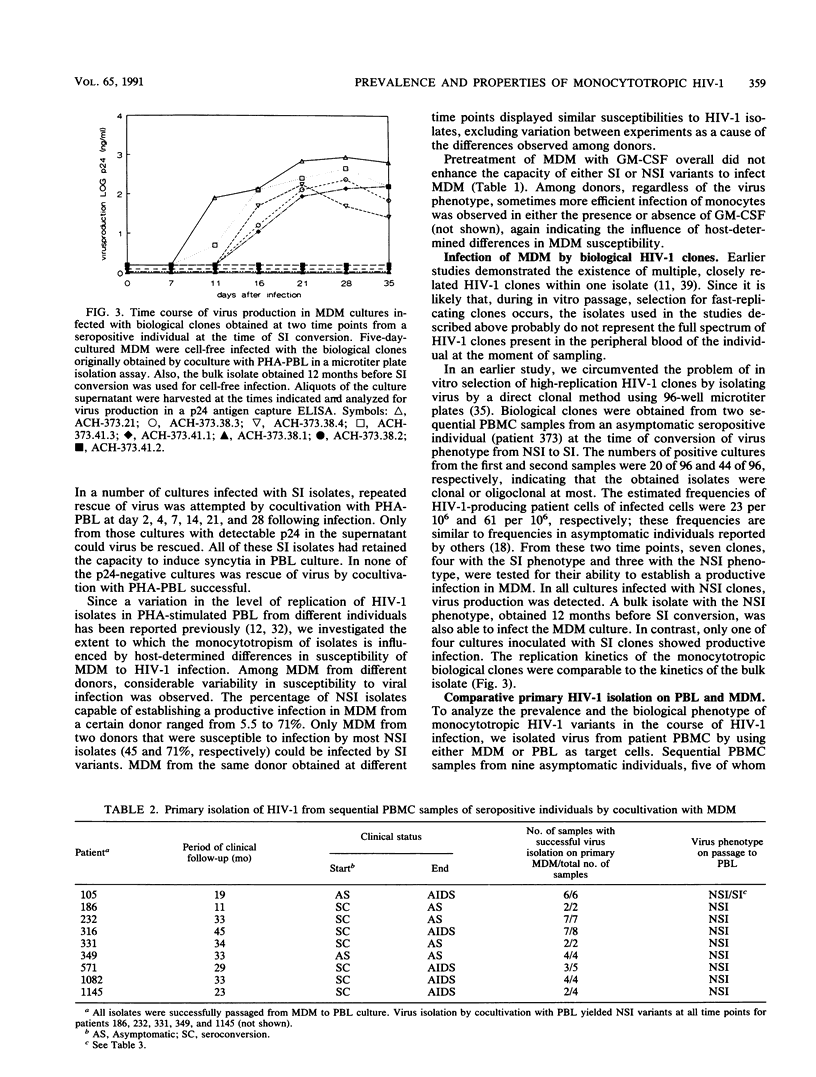
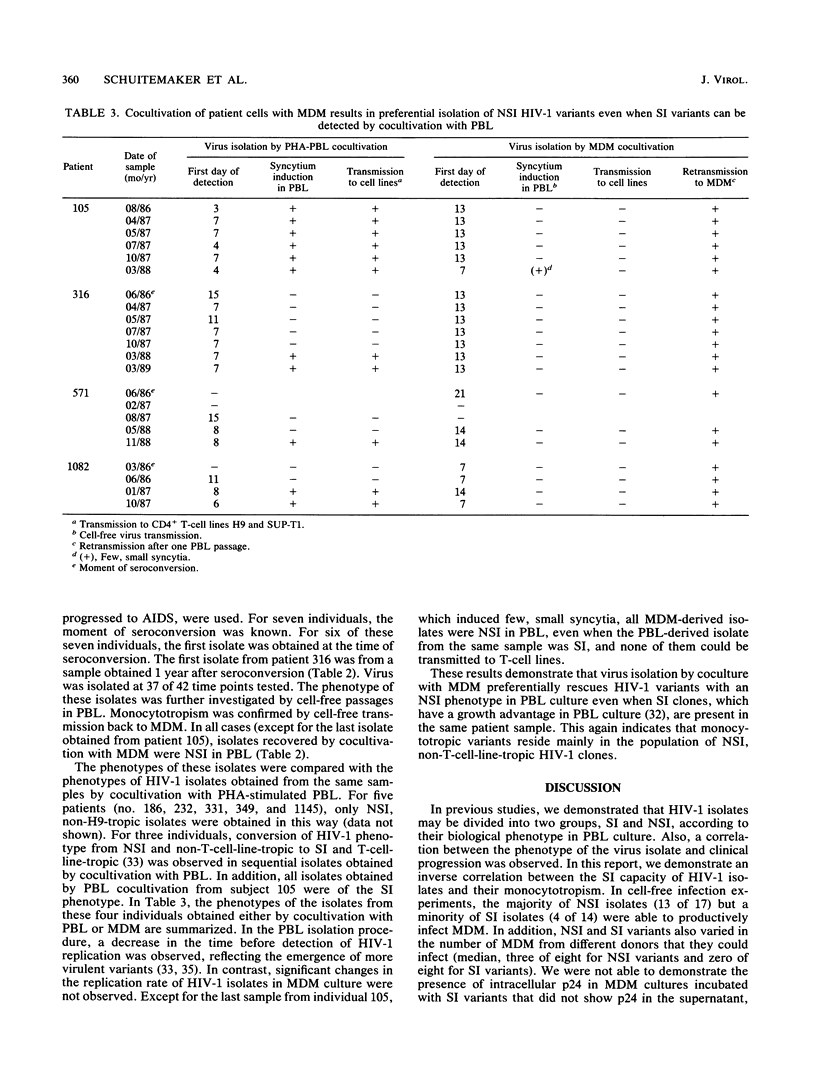
We previously demonstrated a correlation between the presence of syncytium-inducing (SI) human immunodeficiency virus type 1 (HIV-1) variants showing tropism for cell line H9 and the occurrence of rapid CD4 cell decline and progression to AIDS. In contrast, in stable asymptomatic individuals, we detected only isolates with low replication rates that were non-syncytium-inducing (NSI) and nontropic for the H9 cell line. Here, we investigated the monocytotropism of established HIV-1 isolates with a panel of isolates and with biological HIV-1 clones with distinct phenotypes. Moreover, the prevalence and biological phenotypes of monocytotropic HIV-1 variants in the course of HIV-1 infection were analyzed in comparative primary isolation studies on peripheral blood lymphocytes (PBL) and monocyte-derived macrophages (MDM). In cell-free infection studies with MDM from eight blood donors, 13 of 17 NSI isolates but only 4 of 14 SI isolates were able to infect MDM. NSI isolates also infected significantly more different donors than SI variants (median, 3 of 8 versus 0 of 8). This enhanced monocytotropism of NSI isolates was confirmed in experiments with biological HIV-1 clones with distinct phenotypes recovered from the same donor. To investigate the prevalence and biological phenotypes of monocytotropic variants in different stages of HIV-1 infection, sequential isolates from peripheral blood mononuclear cell samples from nine asymptomatic individuals, five of whom progressed to AIDS and seven of whom had a known time of seroconversion, were recovered by cocultivation with both PBL and MDM. Monocytotropic variants were obtained from 37 of 42 time points. All monocytotropic variants were NSI in PBL culture and non-T-cell-line tropic, even when SI, T-cell-line-tropic HIV-1 variants could be recovered from the same patient sample by cocultivation with PBL. We conclude that monocytotropic HIV-1 variants mostly have an NSI phenotype in PBL and, in contrast to SI variants, are present at all stages of HIV-1 infection. These results suggest an important role for monocytotropic variants in the persistence of HIV-1 infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asjö B., Morfeldt-Månson L., Albert J., Biberfeld G., Karlsson A., Lidman K., Fenyö E. M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986 Sep 20;2(8508):660–662. [PubMed] [Google Scholar]
- Baur A., Schwarz N., Ellinger S., Korn K., Harrer T., Mang K., Jahn G. Continuous clearance of HIV in a vertically infected child. Lancet. 1989 Oct 28;2(8670):1045–1045. doi: 10.1016/s0140-6736(89)91061-1. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Homsy J., Evans L. A., Levy J. A. Identification of human immunodeficiency virus subtypes with distinct patterns of sensitivity to serum neutralization. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2815–2819. doi: 10.1073/pnas.85.8.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Tateno M., Levy J. A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988 Apr 1;240(4848):80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Weiss C., Seto D., Levy J. A. Isolates of human immunodeficiency virus type 1 from the brain may constitute a special group of the AIDS virus. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman R., Hassan N. F., Walker R., Godfrey B., Cutilli J., Hastings J. C., Friedman H., Douglas S. D., Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989 Oct 1;170(4):1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. A., McHugh T. M., Stites D. P., Levy J. A. Differential ability of human immunodeficiency virus isolates to productively infect human cells. J Immunol. 1987 May 15;138(10):3415–3418. [PubMed] [Google Scholar]
- Figdor C. G., Bont W. S., Touw I., de Roos J., Roosnek E. E., de Vries J. E. Isolation of functionally different human monocytes by counterflow centrifugation elutriation. Blood. 1982 Jul;60(1):46–53. [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Looney D., Rose A., Gallo R. C., Saag M. S., Shaw G. M., Hahn B. H., Wong-Staal F. Biologically diverse molecular variants within a single HIV-1 isolate. Nature. 1988 Aug 4;334(6181):444–447. doi: 10.1038/334444a0. [DOI] [PubMed] [Google Scholar]
- Folks T., Kelly J., Benn S., Kinter A., Justement J., Gold J., Redfield R., Sell K. W., Fauci A. S. Susceptibility of normal human lymphocytes to infection with HTLV-III/LAV. J Immunol. 1986 Jun 1;136(11):4049–4053. [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Betts R. F., Popovic M. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. JAMA. 1986 Nov 7;256(17):2365–2371. [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Baca L. M., Weiser B., Burger H., Kalter D. C., Meltzer M. S. The macrophage in the persistence and pathogenesis of HIV infection. AIDS. 1989 Aug;3(8):475–495. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Martin M. A., Ferrua C., Mitra R., Phipps T., Wahl L. A., Lane H. C., Fauci A. S., Burke D. S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988 Apr 1;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruters R. A., Terpstra F. G., De Jong R., Van Noesel C. J., Van Lier R. A., Miedema F. Selective loss of T cell functions in different stages of HIV infection. Early loss of anti-CD3-induced T cell proliferation followed by decreased anti-CD3-induced cytotoxic T lymphocyte generation in AIDS-related complex and AIDS. Eur J Immunol. 1990 May;20(5):1039–1044. doi: 10.1002/eji.1830200514. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Moudgil T., Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989 Dec 14;321(24):1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Langlade-Demoyen P., Dadaglio G., Vilmer E., Michel F., Mayaud C., Autran B., Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989 Jan 15;142(2):452–462. [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kornbluth R. S., Oh P. S., Munis J. R., Cleveland P. H., Richman D. D. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J Exp Med. 1989 Mar 1;169(3):1137–1151. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Cross G. D., Callaway C. S., McDougal J. S. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol. 1986 Jul 1;137(1):323–329. [PubMed] [Google Scholar]
- Orenstein J. M., Meltzer M. S., Phipps T., Gendelman H. E. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J Virol. 1988 Aug;62(8):2578–2586. doi: 10.1128/jvi.62.8.2578-2586.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perno C. F., Yarchoan R., Cooney D. A., Hartman N. R., Webb D. S., Hao Z., Mitsuya H., Johns D. G., Broder S. Replication of human immunodeficiency virus in monocytes. Granulocyte/macrophage colony-stimulating factor (GM-CSF) potentiates viral production yet enhances the antiviral effect mediated by 3'-azido-2'3'-dideoxythymidine (AZT) and other dideoxynucleoside congeners of thymidine. J Exp Med. 1989 Mar 1;169(3):933–951. doi: 10.1084/jem.169.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Gartner S. Isolation of HIV-1 from monocytes but not T lymphocytes. Lancet. 1987 Oct 17;2(8564):916–916. doi: 10.1016/s0140-6736(87)91403-6. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Prince A. M., Horowitz B., Baker L., Shulman R. W., Ralph H., Valinsky J., Cundell A., Brotman B., Boehle W., Rey F. Failure of a human immunodeficiency virus (HIV) immune globulin to protect chimpanzees against experimental challenge with HIV. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6944–6948. doi: 10.1073/pnas.85.18.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin S. Z., Rose R. M., Groopman J. E., Markham P. D., Gallo R. C. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986 Jul;68(1):281–284. [PubMed] [Google Scholar]
- Tersmette M., Gruters R. A., de Wolf F., de Goede R. E., Lange J. M., Schellekens P. T., Goudsmit J., Huisman H. G., Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989 May;63(5):2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersmette M., Lange J. M., de Goede R. E., de Wolf F., Eeftink-Schattenkerk J. K., Schellekens P. T., Coutinho R. A., Huisman J. G., Goudsmit J., Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989 May 6;1(8645):983–985. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- Tersmette M., Winkel I. N., Groenink M., Gruters R. A., Spence R. P., Saman E., Van Der Groen G., Miedema F., Huisman J. G. Detection and subtyping of HIV-1 isolates with a panel of characterized monoclonal antibodies to HIV p24gag. Virology. 1989 Jul;171(1):149–155. doi: 10.1016/0042-6822(89)90521-7. [DOI] [PubMed] [Google Scholar]
- Tersmette M., de Goede R. E., Al B. J., Winkel I. N., Gruters R. A., Cuypers H. T., Huisman H. G., Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988 Jun;62(6):2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. S., Stanley S. D., Nastala C. A., Austin A. A., Bartlett J. A., Stine K. C., Lyerly H. K., Bolognesi D. P., Weinhold K. J. Alterations in antibody-dependent cellular cytotoxicity during the course of HIV-1 infection. Humoral and cellular defects. J Immunol. 1990 May 1;144(9):3375–3384. [PubMed] [Google Scholar]
- Weiss S. H., Goedert J. J., Gartner S., Popovic M., Waters D., Markham P., di Marzo Veronese F., Gail M. H., Barkley W. E., Gibbons J. Risk of human immunodeficiency virus (HIV-1) infection among laboratory workers. Science. 1988 Jan 1;239(4835):68–71. doi: 10.1126/science.3336776. [DOI] [PubMed] [Google Scholar]
- Ziza J. M., Brun-Vezinet F., Venet A., Rouzioux C. H., Traversat J., Israel-Biet B., Barre-Sinoussi F., Chermann J. C., Godeau P. Lymphadenopathy-associated virus isolated from bronchoalveolar lavage fluid in AIDS-related complex with lymphoid interstitial pneumonitis. N Engl J Med. 1985 Jul 18;313(3):183–183. doi: 10.1056/NEJM198507183130313. [DOI] [PubMed] [Google Scholar]
- de Wolf F., Lange J. M., Goudsmit J., Cload P., de Gans J., Schellekens P. T., Coutinho R. A., Fiddian A. P., van der Noordaa J. Effect of zidovudine on serum human immunodeficiency virus antigen levels in symptom-free subjects. Lancet. 1988 Feb 20;1(8582):373–376. doi: 10.1016/s0140-6736(88)91179-8. [DOI] [PubMed] [Google Scholar]
- de Wolf F., Lange J. M., Houweling J. T., Coutinho R. A., Schellekens P. T., van der Noordaa J., Goudsmit J. Numbers of CD4+ cells and the levels of core antigens of and antibodies to the human immunodeficiency virus as predictors of AIDS among seropositive homosexual men. J Infect Dis. 1988 Sep;158(3):615–622. doi: 10.1093/infdis/158.3.615. [DOI] [PubMed] [Google Scholar]
- van Noesel C. J., Gruters R. A., Terpstra F. G., Schellekens P. T., van Lier R. A., Miedema F. Functional and phenotypic evidence for a selective loss of memory T cells in asymptomatic human immunodeficiency virus-infected men. J Clin Invest. 1990 Jul;86(1):293–299. doi: 10.1172/JCI114698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Briesen H., Becker W. B., Henco K., Helm E. B., Gelderblom H. R., Brede H. D., Rübsamen-Waigmann H. Isolation frequency and growth properties of HIV-variants: multiple simultaneous variants in a patient demonstrated by molecular cloning. J Med Virol. 1987 Sep;23(1):51–66. doi: 10.1002/jmv.1890230107. [DOI] [PubMed] [Google Scholar]


