Abstract
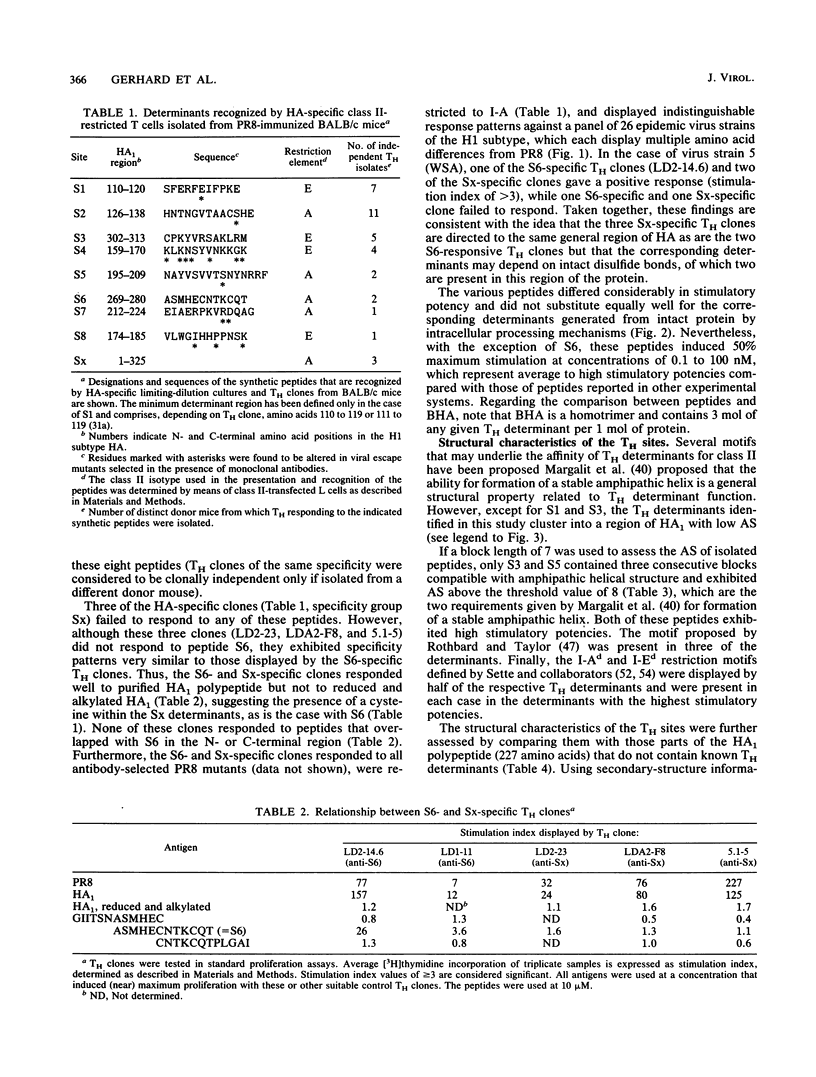
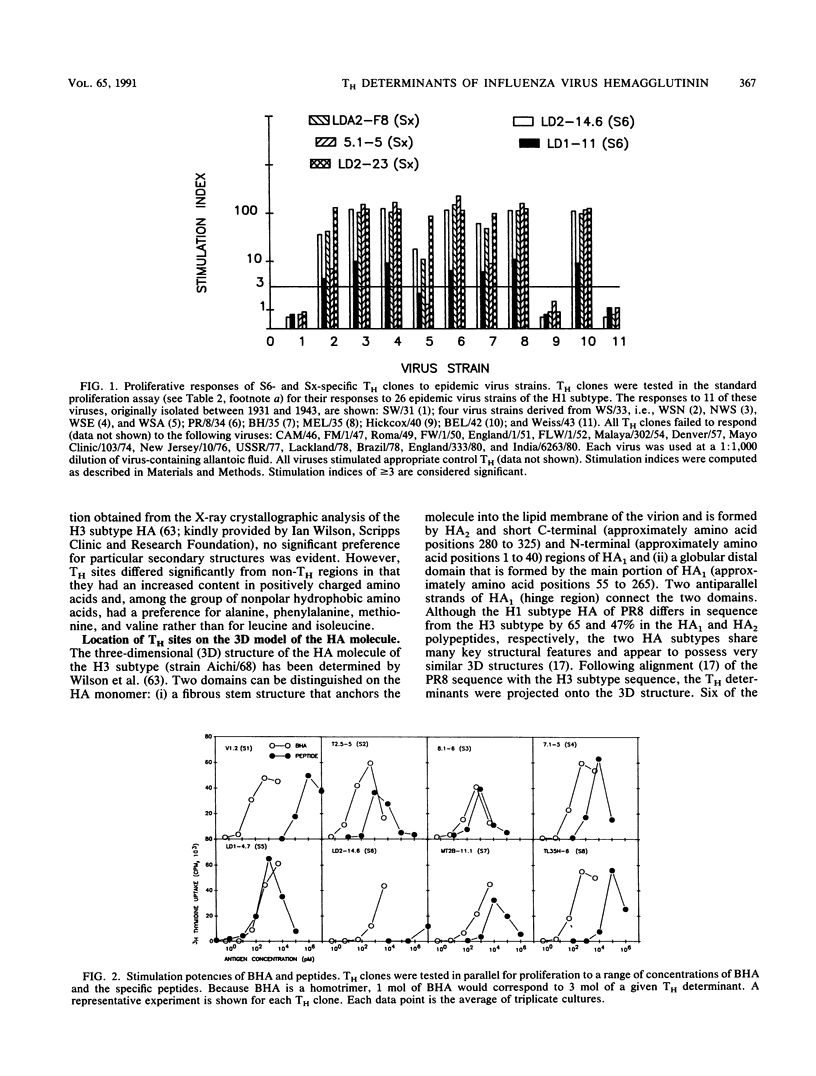
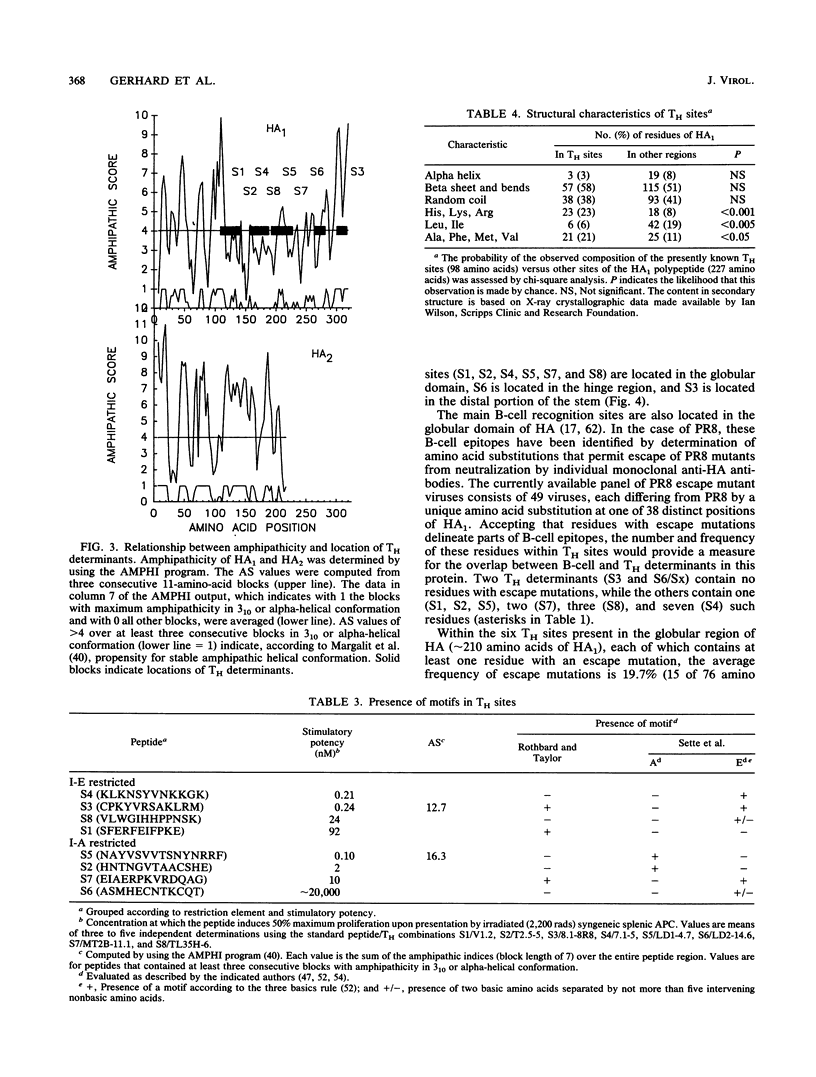
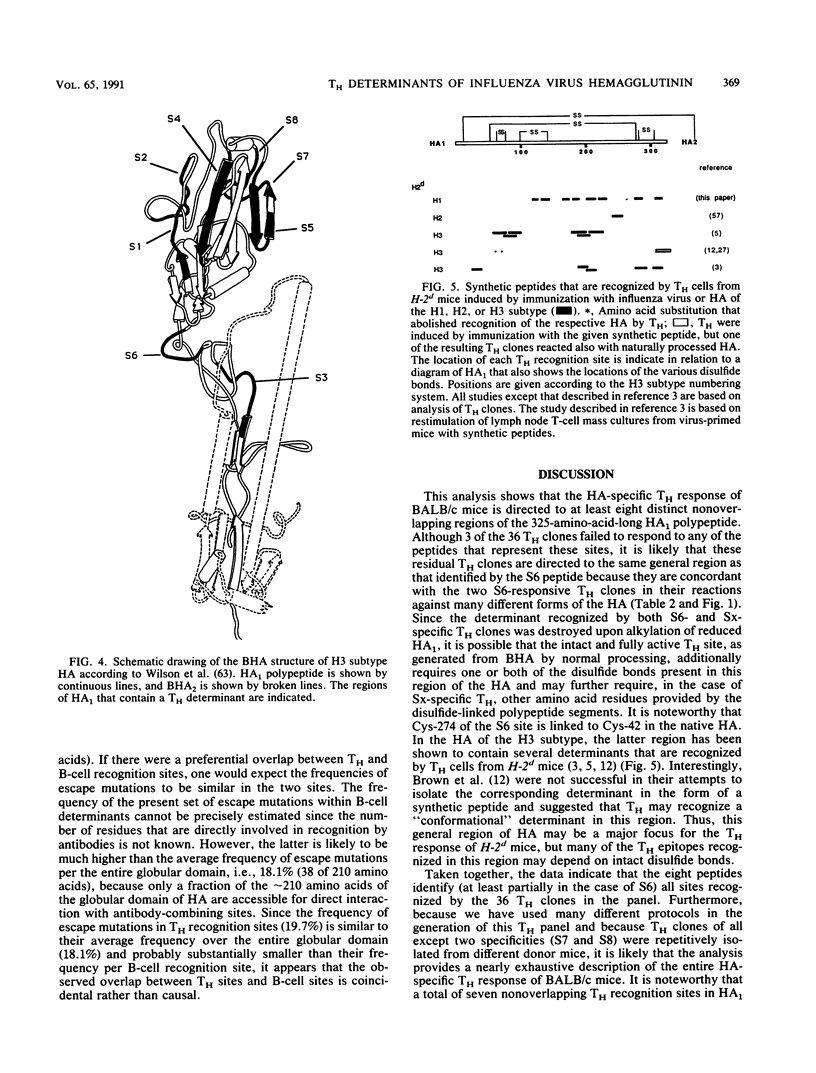
Eight nonoverlapping regions of the hemagglutinin (HA) molecule of influenza virus A/PR/8/34 (PR8), which serve as recognition sites for class II-restricted T cells (TH) from BALB/c mice, have been identified in the form of 10- to 15-amino-acid-long synthetic peptides. These TH determinants are located between residues 110 to 313 of the HA1 polypeptide. From a total of 36 HA-specific TH clones and limiting-dilution cultures of independent clonal origins, 33 (90%) responded to stimulation with one of these peptides. The residual three TH clones appeared to recognize a single additional determinant on the HA1 polypeptide which could not be isolated, however, in the form of a stimulatory peptide. None of the motifs that have been proposed to typify TH determinants were displayed by more than half of these recognition sites. Most unexpected was the finding that none of the TH determinants was located in the ectodomain of the HA2 polypeptide that makes up roughly one-third of the HA molecule. Possible reasons for the preferential recognition of HA1 as opposed to HA2 by TH are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Appella E., Doria G., Nagy Z. A. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988 Dec 1;168(6):2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z., Kurisaki J. A novel approach for localization of the continuous protein antigenic sites by comprehensive synthetic surface scanning: antibody and T-cell activity to several influenza hemagglutinin synthetic sites. Immunol Commun. 1984;13(6):539–551. doi: 10.3109/08820138409061305. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Barnett B. C., Burt D. S., Graham C. M., Warren A. P., Skehel J. J., Thomas D. B. I-Ad restricted T cell recognition of influenza hemagglutinin. Synthetic peptides identify multiple epitopes corresponding to antibody-binding regions of the HA1 subunit. J Immunol. 1989 Oct 15;143(8):2663–2669. [PubMed] [Google Scholar]
- Barnett B. C., Graham C. M., Burt D. S., Skehel J. J., Thomas D. B. The immune response of BALB/c mice to influenza hemagglutinin: commonality of the B cell and T cell repertoires and their relevance to antigenic drift. Eur J Immunol. 1989 Mar;19(3):515–521. doi: 10.1002/eji.1830190316. [DOI] [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A., Brett S. J., Streicher H. Z., Takahashi H. Antigen processing for presentation to T lymphocytes: function, mechanisms, and implications for the T-cell repertoire. Immunol Rev. 1988 Dec;106:5–31. doi: 10.1111/j.1600-065x.1988.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Braciale V. L., Winkler M., Stroynowski I., Hood L., Sambrook J., Gething M. J. On the role of the transmembrane anchor sequence of influenza hemagglutinin in target cell recognition by class I MHC-restricted, hemagglutinin-specific cytolytic T lymphocytes. J Exp Med. 1987 Sep 1;166(3):678–692. doi: 10.1084/jem.166.3.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braciale T. J., Morrison L. A., Sweetser M. T., Sambrook J., Gething M. J., Braciale V. L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987 Aug;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Brown L. E., Ffrench R. A., Gawler J. M., Jackson D. C., Dyall-Smith M. L., Anders E. M., Tregear G. W., Duncan L., Underwood P. A., White D. O. Distinct epitopes recognized by I-Ad-restricted T-cell clones within antigenic site E on influenza virus hemagglutinin. J Virol. 1988 Jan;62(1):305–312. doi: 10.1128/jvi.62.1.305-312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt D. S., Mills K. H., Skehel J. J., Thomas D. B. Diversity of the class II (I-Ak/I-Ek)-restricted T cell repertoire for influenza hemagglutinin and antigenic drift. Six nonoverlapping epitopes on the HA1 subunit are defined by synthetic peptides. J Exp Med. 1989 Aug 1;170(2):383–397. doi: 10.1084/jem.170.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Colon S., Smith C., Freed J. H., Miles C., Grey H. M. Interaction between a "processed" ovalbumin peptide and Ia molecules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3968–3971. doi: 10.1073/pnas.83.11.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Casten L. A., Lakey E. K., Jelachich M. L., Margoliash E., Pierce S. K. Anti-immunoglobulin augments the B-cell antigen-presentation function independently of internalization of receptor-antigen complex. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5890–5894. doi: 10.1073/pnas.82.17.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Antigen presentation by normal B cells, B cell tumors, and macrophages: functional and biochemical comparison. J Immunol. 1982 Apr;128(4):1764–1768. [PubMed] [Google Scholar]
- Davidson H. W., Watts C. Epitope-directed processing of specific antigen by B lymphocytes. J Cell Biol. 1989 Jul;109(1):85–92. doi: 10.1083/jcb.109.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demotz S., Matricardi P. M., Irle C., Panina P., Lanzavecchia A., Corradin G. Processing of tetanus toxin by human antigen-presenting cells. Evidence for donor and epitope-specific processing pathways. J Immunol. 1989 Dec 15;143(12):3881–3886. [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Helenius A., White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem. 1985 Mar 10;260(5):2973–2981. [PubMed] [Google Scholar]
- Eisenlohr L. C., Gerhard W., Hackett C. J. Acid-induced conformational modification of the hemagglutinin molecule alters interaction of influenza virus with antigen-presenting cells. J Immunol. 1988 Sep 15;141(6):1870–1876. [PubMed] [Google Scholar]
- Eisenlohr L. C., Gerhard W., Hackett C. J. Individual class II-restricted antigenic determinants of the same protein exhibit distinct kinetics of appearance and persistence on antigen-presenting cells. J Immunol. 1988 Oct 15;141(8):2581–2584. [PubMed] [Google Scholar]
- Eisenlohr L. C., Gerhard W., Hackett C. J. Role of receptor-binding activity of the viral hemagglutinin molecule in the presentation of influenza virus antigens to helper T cells. J Virol. 1987 May;61(5):1375–1383. doi: 10.1128/jvi.61.5.1375-1383.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr L. C., Hackett C. J. Class II major histocompatibility complex-restricted T cells specific for a virion structural protein that do not recognize exogenous influenza virus. Evidence that presentation of labile T cell determinants is favored by endogenous antigen synthesis. J Exp Med. 1989 Mar 1;169(3):921–931. doi: 10.1084/jem.169.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench R. A., Tang X. L., Anders E. M., Jackson D. C., White D. O., Drummer H., Wade J. D., Tregear G. W., Brown L. E. Class II-restricted T-cell clones to a synthetic peptide of influenza virus hemagglutinin differ in their fine specificities and in the ability to respond to virus. J Virol. 1989 Jul;63(7):3087–3094. doi: 10.1128/jvi.63.7.3087-3094.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon G., Shastri N., Cogswell J., Wilbur S., Sadegh-Nasseri S., Krzych U., Miller A., Sercarz E. The choice of T-cell epitopes utilized on a protein antigen depends on multiple factors distant from, as well as at the determinant site. Immunol Rev. 1987 Aug;98:53–73. doi: 10.1111/j.1600-065x.1987.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Gerhard W., Hackett C., Melchers F. The recognition specificity of a murine helper T cell for hemagglutinin of influenza virus A/PR/8/34. J Immunol. 1983 May;130(5):2379–2385. [PubMed] [Google Scholar]
- Germain R. N., Ashwell J. D., Lechler R. I., Margulies D. H., Nickerson K. M., Suzuki G., Tou J. Y. "Exon-shuffling" maps control of antibody- and T-cell-recognition sites to the NH2-terminal domain of the class II major histocompatibility polypeptide A beta. Proc Natl Acad Sci U S A. 1985 May;82(9):2940–2944. doi: 10.1073/pnas.82.9.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves P. N., Schulman J. L., Young J. F., Palese P. Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: unmasking of cross-reactive HA2 determinants. Virology. 1983 Apr 15;126(1):106–116. doi: 10.1016/0042-6822(83)90465-8. [DOI] [PubMed] [Google Scholar]
- Haberman A. M., Moller C., McCreedy D., Gerhard W. U. A large degree of functional diversity exists among helper T cells specific for the same antigenic site of influenza hemagglutinin. J Immunol. 1990 Nov 1;145(9):3087–3094. [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Katz J. M., Laver W. G., White D. O., Anders E. M. Recognition of influenza virus hemagglutinin by subtype-specific and cross-reactive proliferative T cells: contribution of HA1 and HA2 polypeptide chains. J Immunol. 1985 Jan;134(1):616–622. [PubMed] [Google Scholar]
- Kojima M., Cease K. B., Buckenmeyer G. K., Berzofsky J. A. Limiting dilution comparison of the repertoires of high and low responder MHC-restricted T cells. J Exp Med. 1988 Mar 1;167(3):1100–1113. doi: 10.1084/jem.167.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano K., Braciale T. J., Ennis F. A. Cytotoxic T lymphocytes recognize a cross-reactive epitope on the transmembrane region of influenza H1 and H2 hemagglutinins. Viral Immunol. 1989 Fall;2(3):163–173. doi: 10.1089/vim.1989.2.163. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Green N. Analysis of the antigen specificity of influenza haemagglutinin-immune human T lymphocyte clones: identification of an immunodominant region for T cells. Immunology. 1983 Dec;50(4):659–666. [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Manca F., Fenoglio D., Kunkl A., Cambiaggi C., Sasso M., Celada F. Differential activation of T cell clones stimulated by macrophages exposed to antigen complexed with monoclonal antibodies. A possible influence of paratope specificity on the mode of antigen processing. J Immunol. 1988 May 1;140(9):2893–2898. [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- McElligott D. L., Sorger S. B., Matis L. A., Hedrick S. M. Two distinct mechanisms account for the immune response (Ir) gene control of the T cell response to pigeon cytochrome c. J Immunol. 1988 Jun 15;140(12):4123–4131. [PubMed] [Google Scholar]
- Michalek M. T., Benacerraf B., Rock K. L. Two genetically identical antigen-presenting cell clones display heterogeneity in antigen processing. Proc Natl Acad Sci U S A. 1989 May;86(9):3316–3320. doi: 10.1073/pnas.86.9.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Perkins D. L., Lai M. Z., Smith J. A., Gefter M. L. Identical peptides recognized by MHC class I- and II-restricted T cells. J Exp Med. 1989 Jul 1;170(1):279–289. doi: 10.1084/jem.170.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polydefkis M., Koenig S., Flexner C., Obah E., Gebo K., Chakrabarti S., Earl P. L., Moss B., Siliciano R. F. Anchor sequence-dependent endogenous processing of human immunodeficiency virus 1 envelope glycoprotein gp160 for CD4+ T cell recognition. J Exp Med. 1990 Mar 1;171(3):875–887. doi: 10.1084/jem.171.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B., Abbas A. K. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984 Oct 1;160(4):1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Scherer M. T., Briner T. J., Smith J. A., Gefter M. L. Murine MHC polymorphism and T cell specificities. Science. 1989 May 5;244(4904):572–575. doi: 10.1126/science.2470147. [DOI] [PubMed] [Google Scholar]
- Scherle P. A., Gerhard W. Differential ability of B cells specific for external vs. internal influenza virus proteins to respond to help from influenza virus-specific T-cell clones in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4446–4450. doi: 10.1073/pnas.85.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherle P. A., Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986 Oct 1;164(4):1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Sette A., Adorini L., Appella E., Colón S. M., Miles C., Tanaka S., Ehrhardt C., Doria G., Nagy Z. A., Buus S. Structural requirements for the interaction between peptide antigens and I-Ed molecules. J Immunol. 1989 Nov 15;143(10):3289–3294. [PubMed] [Google Scholar]
- Sette A., Adorini L., Colon S. M., Buus S., Grey H. M. Capacity of intact proteins to bind to MHC class II molecules. J Immunol. 1989 Aug 15;143(4):1265–1267. [PubMed] [Google Scholar]
- Sette A., Buus S., Appella E., Smith J. A., Chesnut R., Miles C., Colon S. M., Grey H. M. Prediction of major histocompatibility complex binding regions of protein antigens by sequence pattern analysis. Proc Natl Acad Sci U S A. 1989 May;86(9):3296–3300. doi: 10.1073/pnas.86.9.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Colon S., Kappler J. W., Marrack P., Grey H. M. Antigen recognition by H-2-restricted T cells. II. A tryptic ovalbumin peptide that substitutes for processed antigen. J Immunol. 1984 Oct;133(4):2067–2074. [PubMed] [Google Scholar]
- Skehel J. J., Bayley P. M., Brown E. B., Martin S. R., Waterfield M. D., White J. M., Wilson I. A., Wiley D. C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982 Feb;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Watari E., Dietzschold B., Szokan G., Heber-Katz E. A synthetic peptide induces long-term protection from lethal infection with herpes simplex virus 2. J Exp Med. 1987 Feb 1;165(2):459–470. doi: 10.1084/jem.165.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Brian A. A., Kappler J. W., Marrack P., McConnell H. M. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7564–7568. doi: 10.1073/pnas.81.23.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdelin O., Mouritsen S., Petersen B. L., Sette A., Buus S. Facts on the fragmentation of antigens in presenting cells, on the association of antigen fragments with MHC molecules in cell-free systems, and speculation on the cell biology of antigen processing. Immunol Rev. 1988 Dec;106:181–193. doi: 10.1111/j.1600-065x.1988.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Heber-Katz E. Lack of immunodominance in the T cell response to herpes simplex virus glycoprotein D after administration of infectious virus. J Exp Med. 1989 Sep 1;170(3):997–1002. doi: 10.1084/jem.170.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]


