Abstract
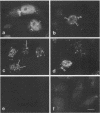
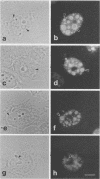
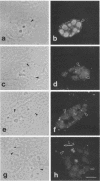
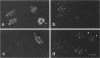
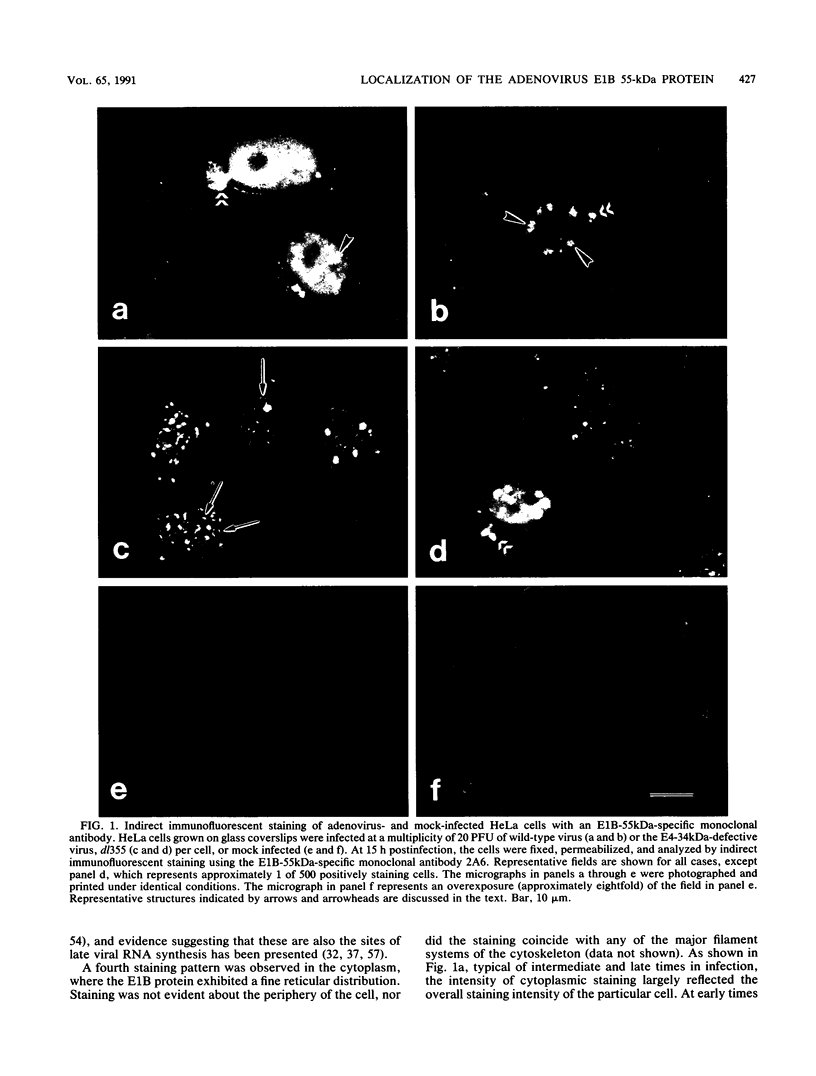
The distribution of the adenovirus early region 1B 55-kDa protein (E1B-55kDa) in lytically infected HeLa cells was determined. At the time of infection, when the E1B-55kDa protein facilitates the cytoplasmic accumulation of viral mRNA while simultaneously restricting the accumulation of most cellular mRNA, five distinct intracellular localizations of the protein were observed. Only one of these was disrupted when cells were infected with a mutant virus that fails to produce a second viral protein encoded by early region 4 (E4-34kDa). This protein normally forms a complex with the E1B-55kDa polypeptide, enabling it to influence RNA metabolism. This key localization of the E1B protein was within and about the periphery of nuclear viral inclusion bodies believed to be the site of viral DNA replication and transcription. In the absence of the E4-34kDa protein, the coincidence of E1B-55kDa-specific immunofluorescence and phase-dense viral inclusions was reduced compared with that in a wild-type infection. Similarly, by immunoelectron microscopy, the relative number of E1B-55kDa-specific immunogold particles associated with the clear fibrillar inclusion bodies was reduced. However, the E4-34kDa protein was not required for the close association of the early region 2A DNA binding protein with the viral inclusions. We propose that the viral 55-kDa-34-kDa protein complex interacts with a cellular factor required for cytoplasmic accumulation of mRNAs and directs it to the periphery of the transcriptionally active viral inclusion bodies. This model provides an explanation for the ability of these viral proteins to simultaneously enhance accumulation of viral mRNAs and inhibit accumulation of cellular mRNAs.
Full text
PDF



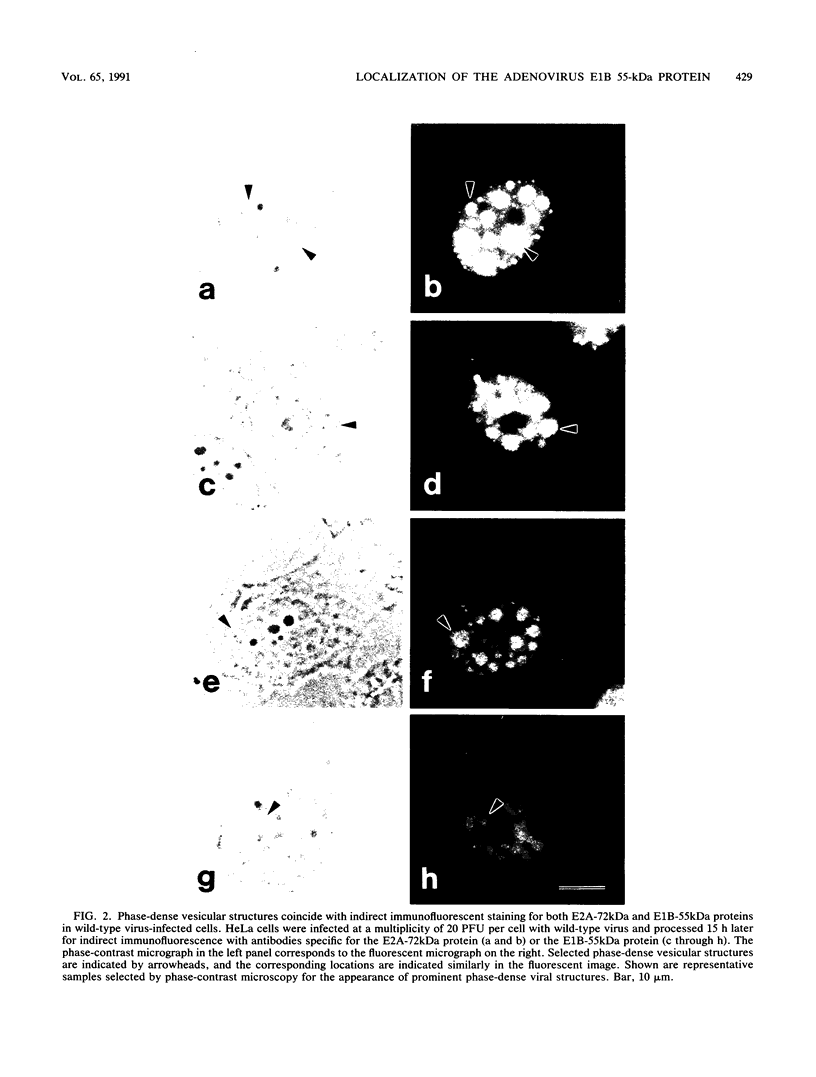
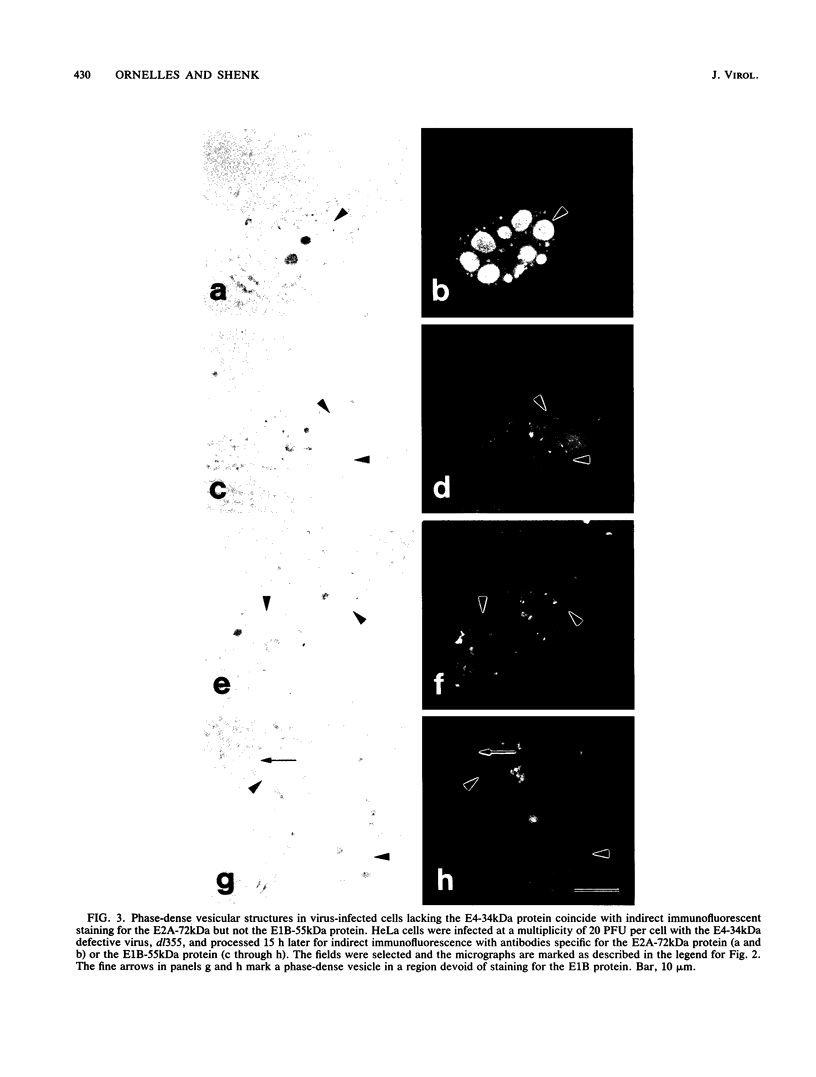
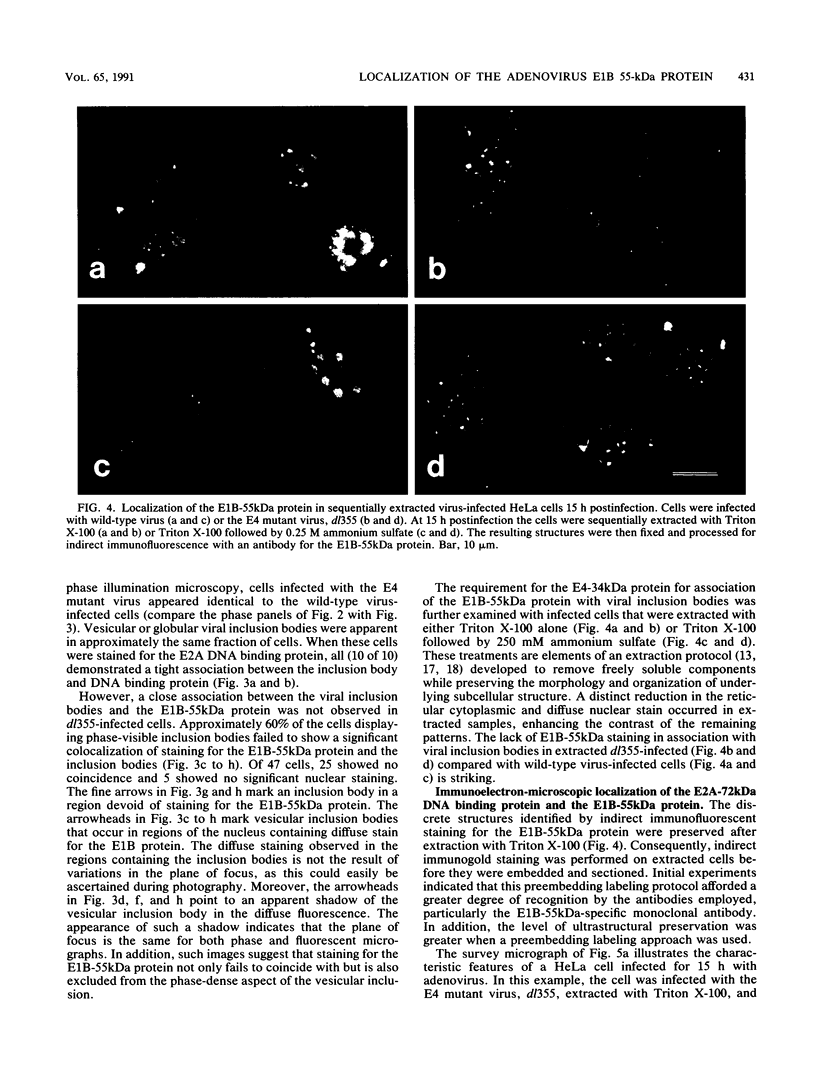
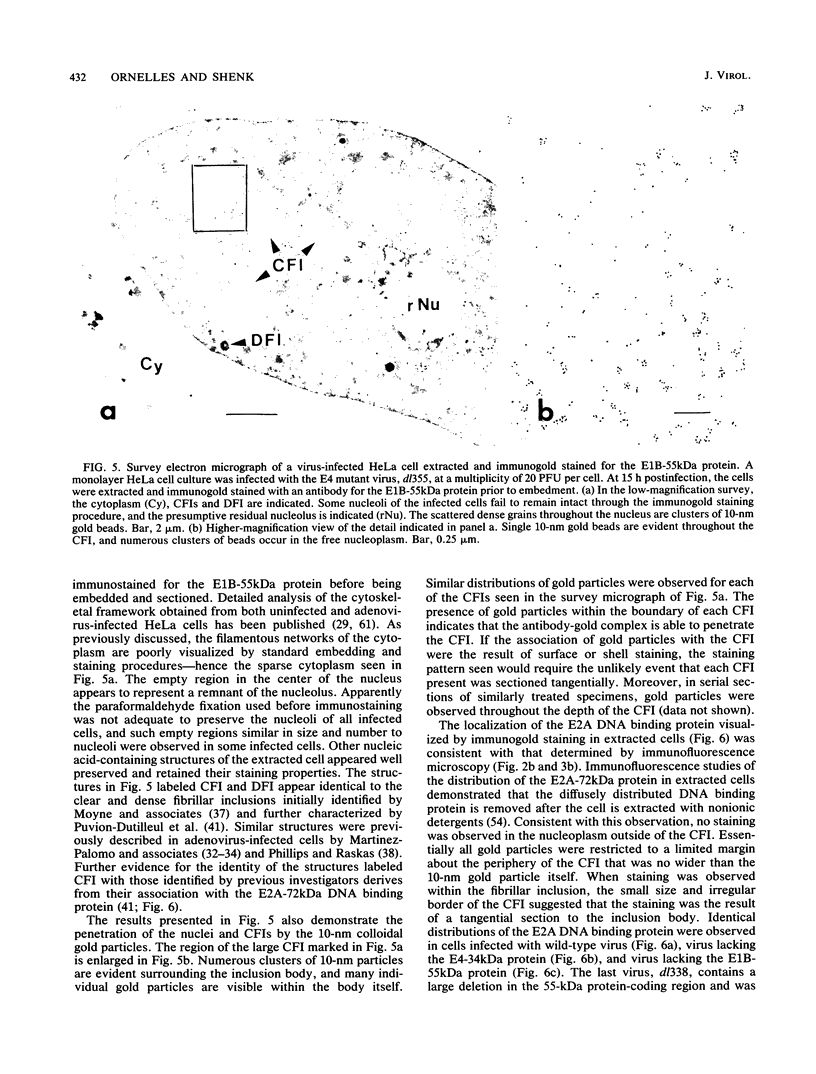






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babich A., Feldman L. T., Nevins J. R., Darnell J. E., Jr, Weinberger C. Effect of adenovirus on metabolism of specific host mRNAs: transport control and specific translational discrimination. Mol Cell Biol. 1983 Jul;3(7):1212–1221. doi: 10.1128/mcb.3.7.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984 Apr;50(1):202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S., Darnell J. E., Jr Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985 Oct;5(10):2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Blair Zajdel M. E., Barker M. D., Dixon S. C., Blair G. E. The use of monoclonal antibodies to study the proteins specified by the transforming region of human adenoviruses. Biochem J. 1985 Feb 1;225(3):649–655. doi: 10.1042/bj2250649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair Zajdel M. E., Blair G. E. The intracellular distribution of the transformation-associated protein p53 in adenovirus-transformed rodent cells. Oncogene. 1988 Jun;2(6):579–584. [PubMed] [Google Scholar]
- Blobel G. Gene gating: a hypothesis. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8527–8529. doi: 10.1073/pnas.82.24.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge E., Ketner G. Interaction of adenoviral E4 and E1b products in late gene expression. Virology. 1990 Feb;174(2):345–353. doi: 10.1016/0042-6822(90)90088-9. [DOI] [PubMed] [Google Scholar]
- Bridge E., Ketner G. Redundant control of adenovirus late gene expression by early region 4. J Virol. 1989 Feb;63(2):631–638. doi: 10.1128/jvi.63.2.631-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Burke B., Griffiths G., Reggio H., Louvard D., Warren G. A monoclonal antibody against a 135-K Golgi membrane protein. EMBO J. 1982;1(12):1621–1628. doi: 10.1002/j.1460-2075.1982.tb01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capco D. G., Krochmalnic G., Penman S. A new method of preparing embeddment-free sections for transmission electron microscopy: applications to the cytoskeletal framework and other three-dimensional networks. J Cell Biol. 1984 May;98(5):1878–1885. doi: 10.1083/jcb.98.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Chaly N., Setterfield G., Kaplan J. G., Brown D. L. Nuclear bodies in mouse splenic lymphocytes: II - Cytochemistry and autoradiography during stimulation by concanavalin A. Biol Cell. 1983;49(1):35–43. doi: 10.1111/j.1768-322x.1984.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Chang L. S., Shenk T. The adenovirus DNA-binding protein stimulates the rate of transcription directed by adenovirus and adeno-associated virus promoters. J Virol. 1990 May;64(5):2103–2109. doi: 10.1128/jvi.64.5.2103-2109.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutt J. R., Shenk T., Hearing P. Analysis of adenovirus early region 4-encoded polypeptides synthesized in productively infected cells. J Virol. 1987 Feb;61(2):543–552. doi: 10.1128/jvi.61.2.543-552.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Wan K. M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984 Jun;98(6):1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Hillman D., Berk A. J. Adenovirus early region 1A protein activates transcription of a nonviral gene introduced into mammalian cells by infection or transfection. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1193–1197. doi: 10.1073/pnas.81.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Gallimore P. H. Adenovirus type 12 early region 1 proteins: a study of their subcellular localization and protein-protein interactions. J Gen Virol. 1984 Dec;65(Pt 12):2149–2166. doi: 10.1099/0022-1317-65-12-2149. [DOI] [PubMed] [Google Scholar]
- Halbert D. N., Cutt J. R., Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985 Oct;56(1):250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D. C., Nickerson J. A., Penman S. Core filaments of the nuclear matrix. J Cell Biol. 1990 Mar;110(3):569–580. doi: 10.1083/jcb.110.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. Sequence-independent autoregulation of the adenovirus type 5 E1A transcription unit. Mol Cell Biol. 1985 Nov;5(11):3214–3221. doi: 10.1128/mcb.5.11.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S. Temperature-sensitive replication of H5ts125 adenovirus DNA in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4291–4295. doi: 10.1073/pnas.75.9.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978 Jan;13(1):181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Khalili K., Weinmann R. Actin mRNAs in HeLa cells. Stabilization after adenovirus infection. J Mol Biol. 1984 Dec 25;180(4):1007–1021. doi: 10.1016/0022-2836(84)90268-7. [DOI] [PubMed] [Google Scholar]
- Lenk R., Storch T., Maizel J. V., Jr Cell architecture during adenovirus infection. Virology. 1980 Aug;105(1):19–34. doi: 10.1016/0042-6822(80)90152-x. [DOI] [PubMed] [Google Scholar]
- Leppard K. N., Shenk T. The adenovirus E1B 55 kd protein influences mRNA transport via an intranuclear effect on RNA metabolism. EMBO J. 1989 Aug;8(8):2329–2336. doi: 10.1002/j.1460-2075.1989.tb08360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner E. A., Lerner M. R., Janeway C. A., Jr, Steitz J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981 May;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Palomo A., Granboulan N. Electron microscopy of adenovirus 12 replication. II. High-resolution autoradiography of infected KB cells labeled with tritiated thymidine. J Virol. 1967 Oct;1(5):1010–1018. doi: 10.1128/jvi.1.5.1010-1018.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Palomo A., Le Buis J., Bernhard W. Electron microscopy of adenovirus 12 replication. 1. Fine structural changes in the nucleus of infected KB cells. J Virol. 1967 Aug;1(4):817–829. doi: 10.1128/jvi.1.4.817-829.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Palomo A. Ultrastructural study of the replication of human adenovirus type 12 in cultured cells. Pathol Microbiol (Basel) 1968;31(3):147–164. doi: 10.1159/000162013. [DOI] [PubMed] [Google Scholar]
- Moffett R. B., Webb T. E. Characterization of a messenger RNA transport protein. Biochim Biophys Acta. 1983 Aug 2;740(3):231–242. doi: 10.1016/0167-4781(83)90131-8. [DOI] [PubMed] [Google Scholar]
- Moore M., Schaack J., Baim S. B., Morimoto R. I., Shenk T. Induced heat shock mRNAs escape the nucleocytoplasmic transport block in adenovirus-infected HeLa cells. Mol Cell Biol. 1987 Dec;7(12):4505–4512. doi: 10.1128/mcb.7.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyne G., Pichard E., Bernhard W. Localization of simian adenovirus 7 (SA 7) transcription and replication in lytic infection. An ultracytochemical and autoradiographical study. J Gen Virol. 1978 Jul;40(1):77–92. doi: 10.1099/0022-1317-40-1-77. [DOI] [PubMed] [Google Scholar]
- Phillips D. M., Raskas H. J. Ultrastructural changes in KB cultures infected with adenovirus type 2. Virology. 1972 Apr;48(1):156–169. doi: 10.1016/0042-6822(72)90123-7. [DOI] [PubMed] [Google Scholar]
- Pilder S., Moore M., Logan J., Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986 Feb;6(2):470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Pédron J., Cajean-Feroldi C. Identification of intranuclear structures containing the 72K DNA-binding protein of human adenovirus type 5. Eur J Cell Biol. 1984 Jul;34(2):313–322. [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Sarnow P., Duprey E., Levine A. J. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology. 1983 Jul 30;128(2):480–484. doi: 10.1016/0042-6822(83)90274-x. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L., Branton P. E. Intracellular localization of adenovirus type 5 tumor antigens in productively infected cells. Virology. 1983 Sep;129(2):456–468. doi: 10.1016/0042-6822(83)90183-6. [DOI] [PubMed] [Google Scholar]
- Sandler A. B., Ketner G. Adenovirus early region 4 is essential for normal stability of late nuclear RNAs. J Virol. 1989 Feb;63(2):624–630. doi: 10.1128/jvi.63.2.624-630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Hearing P., Anderson C. W., Halbert D. N., Shenk T., Levine A. J. Adenovirus early region 1B 58,000-dalton tumor antigen is physically associated with an early region 4 25,000-dalton protein in productively infected cells. J Virol. 1984 Mar;49(3):692–700. doi: 10.1128/jvi.49.3.692-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Sullivan C. A., Levine A. J. A monoclonal antibody detecting the adenovirus type 5-E1b-58Kd tumor antigen: characterization of the E1b-58Kd tumor antigen in adenovirus-infected and -transformed cells. Virology. 1982 Jul 30;120(2):510–517. doi: 10.1016/0042-6822(82)90054-x. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., Deppert W. Specific interaction of simian virus 40 large T antigen with cellular chromatin and nuclear matrix during the course of infection. J Virol. 1987 Nov;61(11):3561–3569. doi: 10.1128/jvi.61.11.3561-3569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. K., Young M. A., Flint S. J. Intranuclear location of the adenovirus type 5 E1B 55-kilodalton protein. J Virol. 1990 Sep;64(9):4558–4564. doi: 10.1128/jvi.64.9.4558-4564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam A., Thirunavukkarasu M., Rajamanickam C. Role of cytosol in the stimulation of RNA transport in vitro during cardiac hypertrophy in rats. Biochem J. 1990 Apr 1;267(1):133–140. doi: 10.1042/bj2670133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara K., Gilead Z., Wold W. S., Green M. Immunofluorescence study of the adenovirus type 2 single-stranded DNA binding protein in infected and transformed cells. J Virol. 1977 May;22(2):527–539. doi: 10.1128/jvi.22.2.527-539.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliet P. C., Sussenbach J. S. An adenovirus type 5 gene function required for initiation of viral DNA replication. Virology. 1975 Oct;67(2):415–426. doi: 10.1016/0042-6822(75)90443-2. [DOI] [PubMed] [Google Scholar]
- Voelkerding K., Klessig D. F. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA-binding protein. J Virol. 1986 Nov;60(2):353–362. doi: 10.1128/jvi.60.2.353-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D. H., Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J Virol. 1986 Mar;57(3):833–838. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Spector D., Welch W. Differential distribution of the adenovirus E1A proteins and colocalization of E1A with the 70-kilodalton cellular heat shock protein in infected cells. J Virol. 1988 Nov;62(11):4153–4166. doi: 10.1128/jvi.62.11.4153-4166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth D. J., Hsu M. T. Visualization of nascent RNA transcripts and simultaneous transcription and replication in viral nucleoprotein complexes from adenovirus 2-infected HeLa cells. J Mol Biol. 1981 Apr 5;147(2):247–268. doi: 10.1016/0022-2836(81)90440-x. [DOI] [PubMed] [Google Scholar]
- Wolosewick J. J. The application of polyethylene glycol (PEG) to electron microscopy. J Cell Biol. 1980 Aug;86(2):675–661. doi: 10.1083/jcb.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zantema A., Fransen J. A., Davis-Olivier A., Ramaekers F. C., Vooijs G. P., DeLeys B., Van der Eb A. J. Localization of the E1B proteins of adenovirus 5 in transformed cells, as revealed by interaction with monoclonal antibodies. Virology. 1985 Apr 15;142(1):44–58. doi: 10.1016/0042-6822(85)90421-0. [DOI] [PubMed] [Google Scholar]
- Zantema A., Schrier P. I., Davis-Olivier A., van Laar T., Vaessen R. T., van der EB A. J. Adenovirus serotype determines association and localization of the large E1B tumor antigen with cellular tumor antigen p53 in transformed cells. Mol Cell Biol. 1985 Nov;5(11):3084–3091. doi: 10.1128/mcb.5.11.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z. H., Nickerson J. A., Krochmalnic G., Penman S. Alterations in nuclear matrix structure after adenovirus infection. J Virol. 1987 Apr;61(4):1007–1018. doi: 10.1128/jvi.61.4.1007-1018.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]