Abstract
In adult rats infected with Borna disease virus, the virus was found exclusively in the brain, whereas in cyclosporine A-treated rats, infectious virus was also detected in peripheral nerve fibers and, unexpectedly, in adjacent organ-specific cells. In contrast to untreated virus-infected rats, no major histocompatibility complex class II expression was found in the brain of cyclosporine A-treated animals.
Full text
PDF
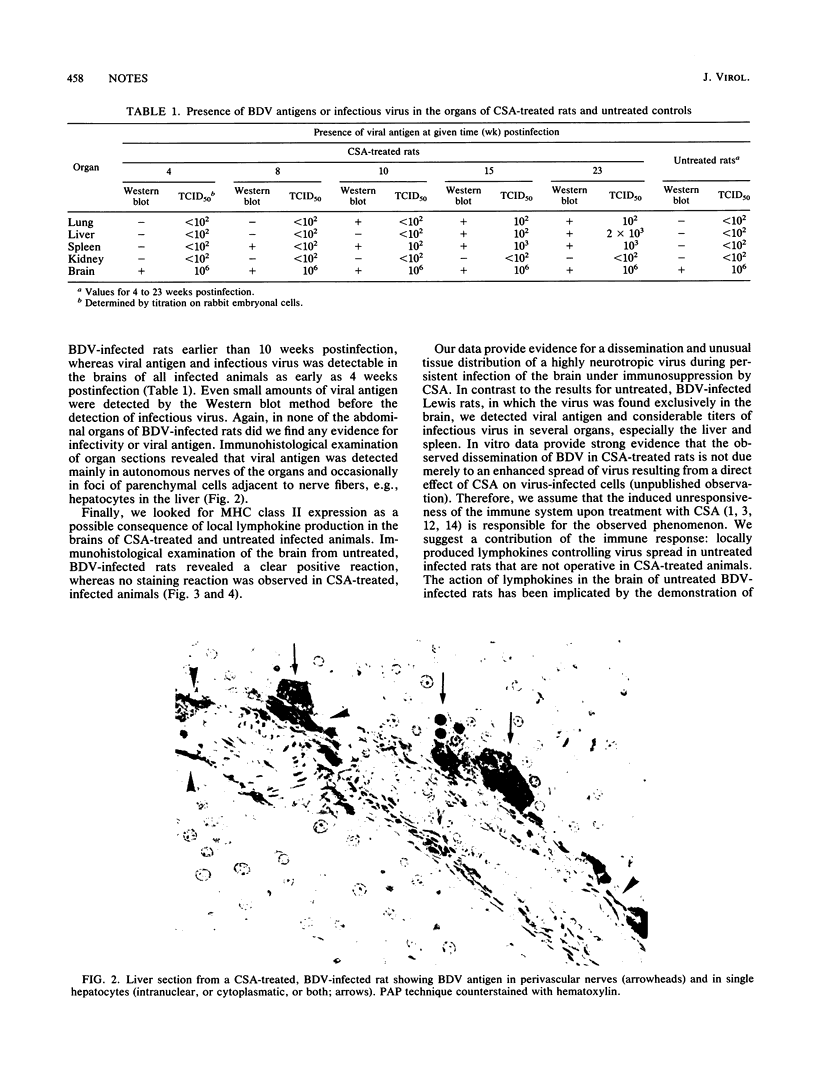
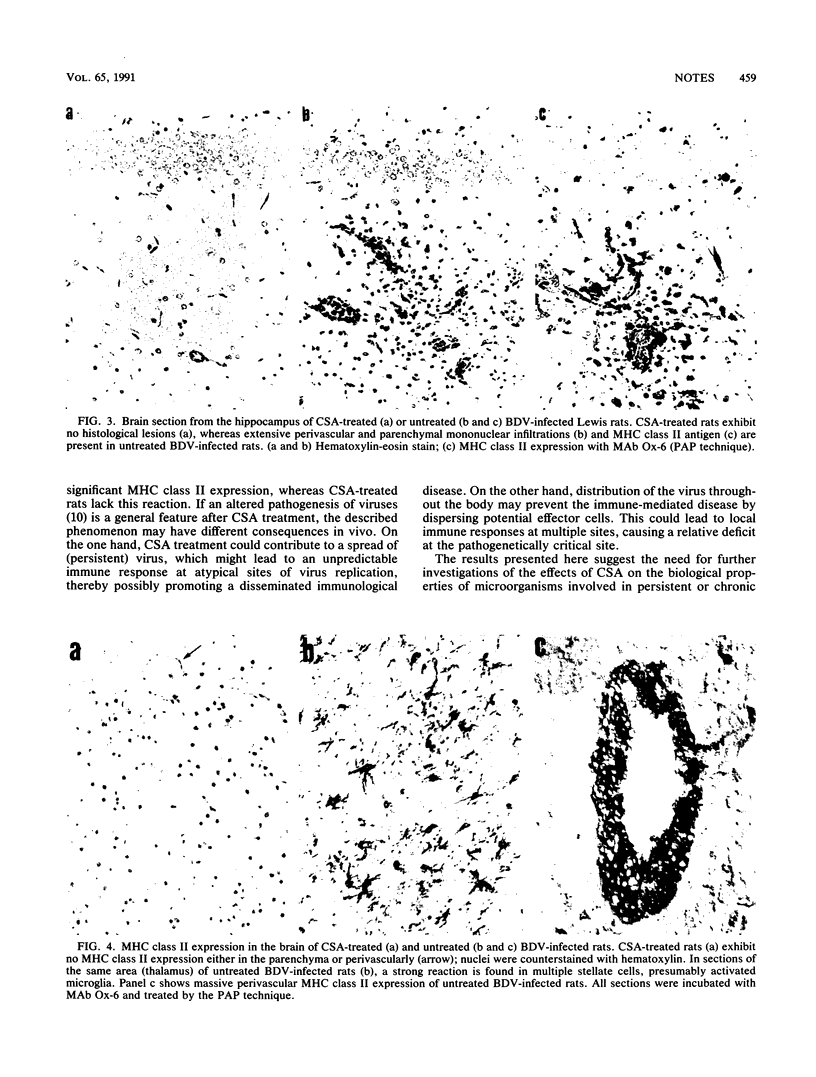

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Carbone K. M., Trapp B. D., Griffin J. W., Duchala C. S., Narayan O. Astrocytes and Schwann cells are virus-host cells in the nervous system of rats with Borna disease. J Neuropathol Exp Neurol. 1989 Nov;48(6):631–644. doi: 10.1097/00005072-198911000-00005. [DOI] [PubMed] [Google Scholar]
- Espevik T., Figari I. S., Shalaby M. R., Lackides G. A., Lewis G. D., Shepard H. M., Palladino M. A., Jr Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987 Aug 1;166(2):571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog S., Kompter C., Frese K., Rott R. Replication of Borna disease virus in rats: age-dependent differences in tissue distribution. Med Microbiol Immunol. 1984;173(4):171–177. doi: 10.1007/BF02122108. [DOI] [PubMed] [Google Scholar]
- Herzog S., Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168(3):153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- Hirano N., Kao M., Ludwig H. Persistent, tolerant or subacute infection in Borna disease virus-infected rats. J Gen Virol. 1983 Jul;64(Pt 7):1521–1530. doi: 10.1099/0022-1317-64-7-1521. [DOI] [PubMed] [Google Scholar]
- Kao M., Ludwig H., Gosztonyi G. Adaptation of Borna disease virus to the mouse. J Gen Virol. 1984 Oct;65(Pt 10):1845–1849. doi: 10.1099/0022-1317-65-10-1845. [DOI] [PubMed] [Google Scholar]
- Ludwig H., Bode L., Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- Ludwig H., Koester V., Pauli G., Rott R. The cerebrospinal fluid of rabbits infected with Borna disease virus. Arch Virol. 1977;55(3):209–223. doi: 10.1007/BF01319907. [DOI] [PubMed] [Google Scholar]
- Markham D. F., Jr, Griffith B. P., Lerner E., Lucia H. L., Bia F. J. Effects of cyclosporine on chronic cytomegalovirus infection in the guinea pig. Intervirology. 1987;28(3):171–180. doi: 10.1159/000150013. [DOI] [PubMed] [Google Scholar]
- Narayan O., Herzog S., Frese K., Scheefers H., Rott R. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J Infect Dis. 1983 Aug;148(2):305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- Palacios R., Martinez-Maza O., De Ley M. Production of human immune interferon (Hu IFN-gamma) studied at the single cell level. Origin, evidence for spontaneous secretion and effect of cyclosporin A. Eur J Immunol. 1983 Mar;13(3):221–225. doi: 10.1002/eji.1830130308. [DOI] [PubMed] [Google Scholar]
- Richt J. A., Stitz L., Wekerle H., Rott R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J Exp Med. 1989 Sep 1;170(3):1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Stitz L., Hengartner H., Althage A., Zinkernagel R. M. An easy and rapid method to screen large numbers of antibodies against internal cellular determinants. J Immunol Methods. 1988 Feb 10;106(2):211–216. doi: 10.1016/0022-1759(88)90199-8. [DOI] [PubMed] [Google Scholar]
- Stitz L., Krey H., Ludwig H. Borna disease in rhesus monkeys as a models for uveo-cerebral symptoms. J Med Virol. 1981;6(4):333–340. doi: 10.1002/jmv.1890060408. [DOI] [PubMed] [Google Scholar]
- Stitz L., Soeder D., Deschl U., Frese K., Rott R. Inhibition of immune-mediated meningoencephalitis in persistently Borna disease virus-infected rats by cyclosporine A. J Immunol. 1989 Dec 15;143(12):4250–4256. [PubMed] [Google Scholar]
- Waelchli R. O., Ehrensperger F., Metzler A., Winder C. Borna disease in a sheep. Vet Rec. 1985 Nov 9;117(19):499–500. doi: 10.1136/vr.117.19.499. [DOI] [PubMed] [Google Scholar]