Abstract
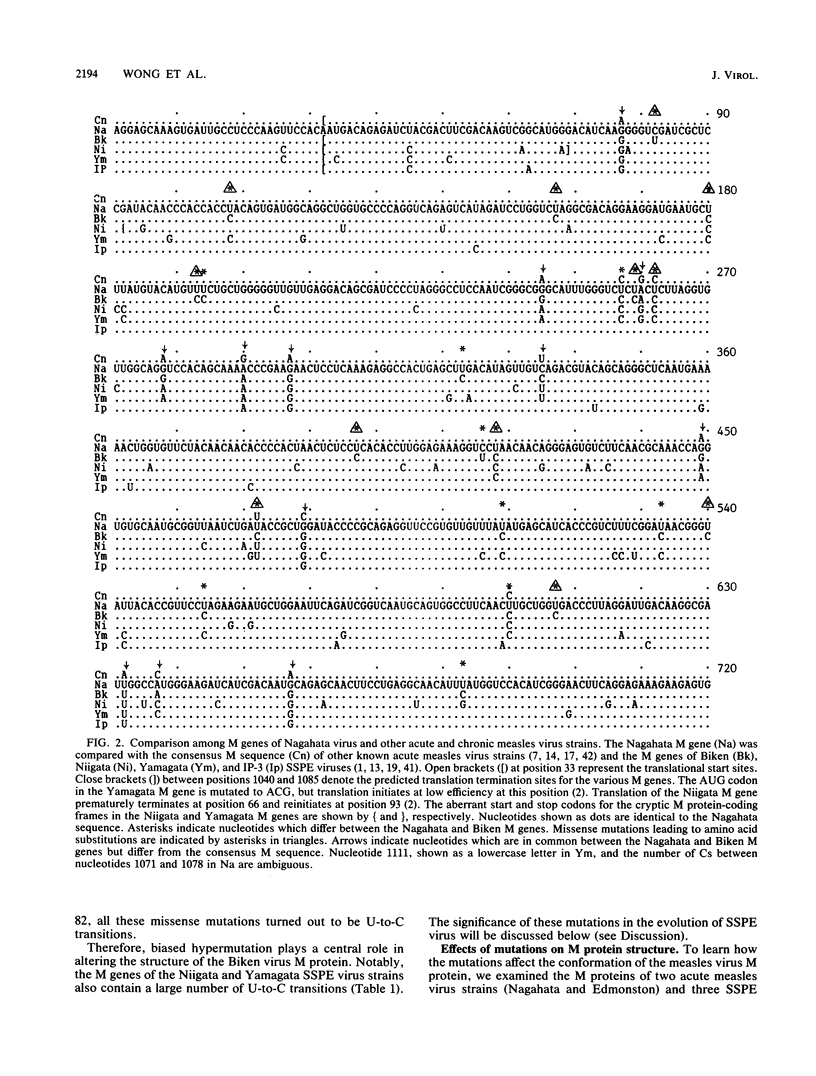
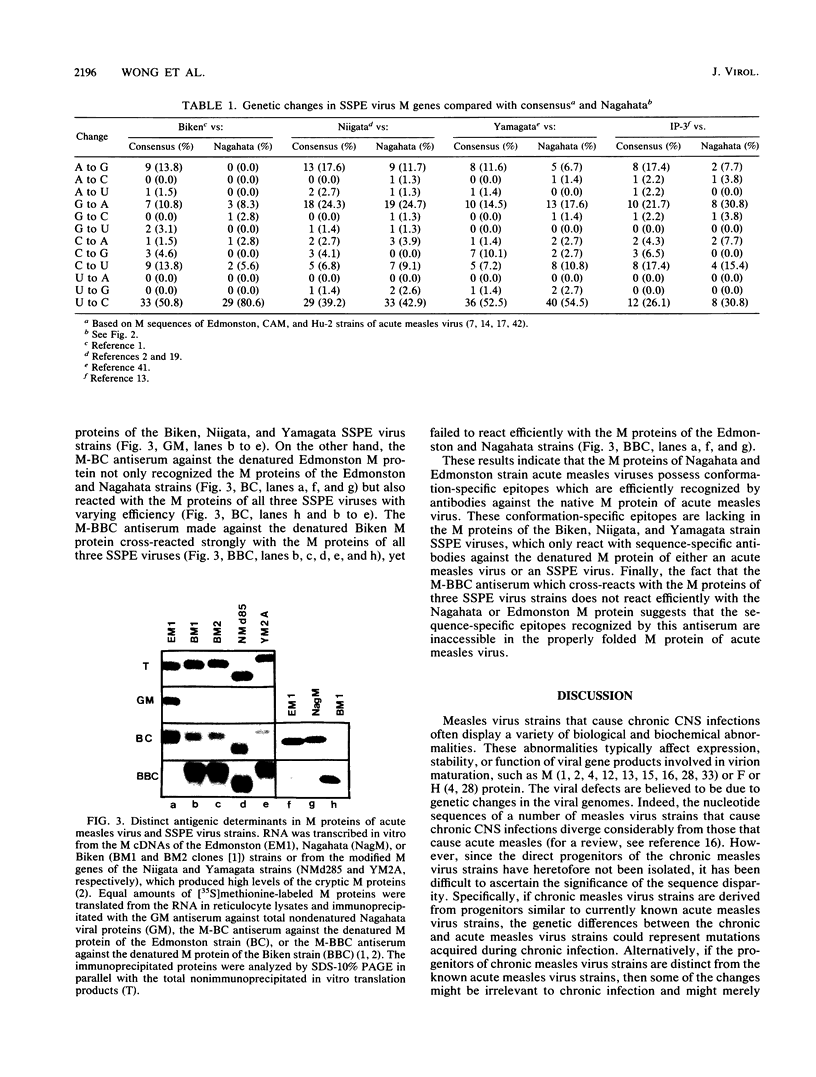
We identified an acute measles virus (Nagahata strain) closely related to a defective virus (Biken strain) isolated from a patient with subacute sclerosing panencephalitis (SSPE). The proteins of Nagahata strain measles virus are antigenically and electrophoretically similar to the proteins of Edmonston strain measles virus. However, the nucleotide sequence of the Nagahata matrix (M) gene is significantly different from the M genes of all the acute measles virus strains studied to date. The Nagahata M gene is strikingly similar to the M gene of Biken strain SSPE virus isolated several years later in the same locale. Eighty percent of the nucleotide differences between the Nagahata and Biken M genes are uridine-to-cytosine transitions known as biased hypermutation, which has been postulated to be caused by a cellular RNA-modifying activity. These biased mutations account for all but one of the numerous missense genetic changes predicted to cause amino acid substitutions. As a result, the Biken virus M protein loses conformation-specific epitopes that are conserved in the M proteins of Nagahata and Edmonston strain acute measles viruses. These conformation-specific epitopes are also absent in the cryptic M proteins encoded by the hypermutated M genes of two other defective SSPE viruses (Niigata and Yamagata strains). Nagahata-like sequences are found in the M genes of at least five other SSPE viruses isolated from three continents. These data indicate that Biken strain SSPE virus is derived from a progenitor closely resembling Nagahata strain acute measles virus and that biased hypermutation is largely responsible for the structural defects in the Biken virus M protein.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayata M., Hirano A., Wong T. C. Altered translation of the matrix genes in Niigata and Yamagata neurovirulent measles virus strains. Virology. 1991 Jan;180(1):166–174. doi: 10.1016/0042-6822(91)90020-c. [DOI] [PubMed] [Google Scholar]
- Ayata M., Hirano A., Wong T. C. Structural defect linked to nonrandom mutations in the matrix gene of biken strain subacute sclerosing panencephalitis virus defined by cDNA cloning and expression of chimeric genes. J Virol. 1989 Mar;63(3):1162–1173. doi: 10.1128/jvi.63.3.1162-1173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Carter M. J., Billeter M., ter Meulen V. Measles virus gene expression in subacute sclerosing panencephalitis. Virus Res. 1984 Oct;1(7):585–595. doi: 10.1016/0168-1702(84)90015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Billeter M., Cattaneo R., Budka H., ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986 Aug;59(2):472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988 Dec 23;55(6):1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H., Cattaneo R., Billeter M. A. Biased hypermutation of viral RNA genomes could be due to unwinding/modification of double-stranded RNA. Cell. 1989 Feb 10;56(3):331–331. doi: 10.1016/0092-8674(89)90234-1. [DOI] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Richardson C. D., Rozenblatt S., Lazzarini R. A. Matrix genes of measles virus and canine distemper virus: cloning, nucleotide sequences, and deduced amino acid sequences. J Virol. 1986 May;58(2):408–416. doi: 10.1128/jvi.58.2.408-416.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland R., Gerald C., Barker D., Wild F. Cloning and sequencing of the nucleoprotein gene of measles virus (Hallé strain). Nucleic Acids Res. 1988 Dec 23;16(24):11821–11821. doi: 10.1093/nar/16.24.11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland R., Gerald C., Barker R., Wild T. F. Fusion glycoprotein of measles virus: nucleotide sequence of the gene and comparison with other paramyxoviruses. J Gen Virol. 1987 Jun;68(Pt 6):1695–1703. doi: 10.1099/0022-1317-68-6-1695. [DOI] [PubMed] [Google Scholar]
- Burnstein T., Jacobsen L. B., Zeman W., Chen T. T. Persistent infection of BSC-1 cells by defective measles virus derived from subacute sclerosing panencephalitis. Infect Immun. 1974 Dec;10(6):1378–1382. doi: 10.1128/iai.10.6.1378-1382.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., ter Meulen V. Defective translation of measles virus matrix protein in a subacute sclerosing panencephalitis cell line. Nature. 1983 Sep 8;305(5930):153–155. doi: 10.1038/305153a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Billeter M. A., Sheppard R. D., Udem S. A. Multiple viral mutations rather than host factors cause defective measles virus gene expression in a subacute sclerosing panencephalitis cell line. J Virol. 1988 Apr;62(4):1388–1397. doi: 10.1128/jvi.62.4.1388-1397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Eschle D., Baczko K., ter Meulen V., Billeter M. A. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988 Oct 21;55(2):255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Rebmann G., Baczko K., Ter Meulen V., Bellini W. J., Rozenblatt S., Billeter M. A. Accumulated measles virus mutations in a case of subacute sclerosing panencephalitis: interrupted matrix protein reading frame and transcription alteration. Virology. 1986 Oct 15;154(1):97–107. doi: 10.1016/0042-6822(86)90433-2. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Spielhofer P., Kaelin K., Baczko K., ter Meulen V., Pardowitz J., Flanagan S., Rima B. K., Udem S. A. Mutated and hypermutated genes of persistent measles viruses which caused lethal human brain diseases. Virology. 1989 Dec;173(2):415–425. doi: 10.1016/0042-6822(89)90554-0. [DOI] [PubMed] [Google Scholar]
- Curran M. D., Rima B. K. Nucleotide sequence of the gene encoding the matrix protein of a recent measles virus isolate. J Gen Virol. 1988 Sep;69(Pt 9):2407–2411. doi: 10.1099/0022-1317-69-9-2407. [DOI] [PubMed] [Google Scholar]
- Doi Y., Sanpe T., Nakajima M., Okawa S., Koto T. Properties of a cytopathic agent isolated from a patient with subacute sclerosing panencephalitis in Japan. Jpn J Med Sci Biol. 1972 Oct;25(5):321–333. doi: 10.7883/yoken1952.25.321. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F., KATZ S. L., HOLLOWAY A. Development of attenuated measles-virus vaccines. A summary of recentinvestigation. Am J Dis Child. 1962 Mar;103:335–340. doi: 10.1001/archpedi.1962.02080020347030. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F., PEEBLES T. C. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc Soc Exp Biol Med. 1954 Jun;86(2):277–286. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- Enami M., Sato T. A., Sugiura A. Matrix protein of cell-associated subacute sclerosing panencephalitis viruses. J Gen Virol. 1989 Aug;70(Pt 8):2191–2196. doi: 10.1099/0022-1317-70-8-2191. [DOI] [PubMed] [Google Scholar]
- Gerald C., Buckland R., Barker R., Freeman G., Wild T. F. Measles virus haemagglutinin gene: cloning, complete nucleotide sequence analysis and expression in COS cells. J Gen Virol. 1986 Dec;67(Pt 12):2695–2703. doi: 10.1099/0022-1317-67-12-2695. [DOI] [PubMed] [Google Scholar]
- Homma M., Tashiro M., Konno H., Ohara Y., Hino M., Takase S. Isolation and characterization of subacute sclerosing panencephalitis virus (Yamagata-1 strain) from a brain autopsy. Microbiol Immunol. 1982;26(12):1195–1202. doi: 10.1111/j.1348-0421.1982.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Hamilton R., Wittig B., Fuccillo D. A., Sever J. L., Vernon M. L. Subacute sclerosing panencephalitis: isolation of suppressed measles virus from lymph node biopsies. Science. 1971 Aug 27;173(3999):840–841. doi: 10.1126/science.173.3999.840. [DOI] [PubMed] [Google Scholar]
- Kobune K., Kobune F., Yamanouchi K., Nagashima K., Yoshikawa Y., Hayami M. Neurovirulence of rat brain-adapted measles virus. Jpn J Exp Med. 1983 Jun;53(3):177–180. [PubMed] [Google Scholar]
- Kratzsch V., Hall W. W., Nagashima K., ter Meulen V. Biological and biochemical characterization of a latent subacute sclerosing panencephalitis (SSPE) virus infection in tissue culture. J Med Virol. 1977;1(2):139–154. doi: 10.1002/jmv.1890010207. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Norrby E. Persistence of RNA viruses in the central nervous system. Annu Rev Microbiol. 1986;40:159–184. doi: 10.1146/annurev.mi.40.100186.001111. [DOI] [PubMed] [Google Scholar]
- Liebert U. G., Baczko K., Budka H., ter Meulen V. Restricted expression of measles virus proteins in brains from cases of subacute sclerosing panencephalitis. J Gen Virol. 1986 Nov;67(Pt 11):2435–2444. doi: 10.1099/0022-1317-67-11-2435. [DOI] [PubMed] [Google Scholar]
- Mawhinney H., Allen I. V., Beare J. M., Bridges J. M., Connolly J. H., Haire M., Nevin N. C., Neill D. W., Hobbs J. R. Dysgammaglobulinaemia complicated by disseminated measles. Br Med J. 1971 May 15;2(5758):380–381. doi: 10.1136/bmj.2.5758.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L., Beffy P., Portner A. Restriction of cell surface expression of Sendai virus hemagglutinin-neuraminidase glycoprotein correlates with its higher instability in persistently and standard plus defective interfering virus infected BHK-21 cells. Virology. 1984 Oct 15;138(1):118–128. doi: 10.1016/0042-6822(84)90152-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard R. D., Raine C. S., Bornstein M. B., Udem S. A. Rapid degradation restricts measles virus matrix protein expression in a subacute sclerosing panencephalitis cell line. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7913–7917. doi: 10.1073/pnas.83.20.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Okuno Y., Hamamoto Y., Oya H. Subacute sclerosing panencephalitis (SSPE): isolation of a defective variant of measles virus from brain obtained at autopsy. Biken J. 1975 Jun;18(2):113–122. [PubMed] [Google Scholar]
- Ueda S., Takahashi M., Kurimura T., Minekawa Y., Suzuki N. Development of extremely attenuated live measles virus vaccine (CAM-EX). Biken J. 1972 Sep;15(3):173–177. [PubMed] [Google Scholar]
- Ueda S., Takahashi M., Minekawa Y., Ogino T., Suzuki N. Studies on further attenuated live measles vaccine. I. Adaptation of measles virus to the chorioallantoic membrane of chick embryo and clinical tests on the strain. Biken J. 1970 Jun;13(2):111–116. [PubMed] [Google Scholar]
- Wagner R. W., Smith J. E., Cooperman B. S., Nishikura K. A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2647–2651. doi: 10.1073/pnas.86.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Meissner H. C. Measles and SSPE viruses: similarities and differences. Prog Med Virol. 1982;28:65–95. [PubMed] [Google Scholar]
- Wong T. C., Ayata M., Hirano A., Yoshikawa Y., Tsuruoka H., Yamanouchi K. Generalized and localized biased hypermutation affecting the matrix gene of a measles virus strain that causes subacute sclerosing panencephalitis. J Virol. 1989 Dec;63(12):5464–5468. doi: 10.1128/jvi.63.12.5464-5468.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Wipf G., Hirano A. The measles virus matrix gene and gene product defined by in vitro and in vivo expression. Virology. 1987 Apr;157(2):497–508. doi: 10.1016/0042-6822(87)90292-3. [DOI] [PubMed] [Google Scholar]