Abstract
Lymphoid tissues from asymptomatic HIV-infected individuals, as compared with symptomatic HIV-infected subjects, show limited histopathological changes and lower levels of HIV expression. In this report we correlate the control of HIV replication in lymph nodes to the non-cytolytic anti-HIV activity of lymphoid tissue CD8+ cells. Five subjects at different stages of HIV-related disease were studied and the ability of their CD8+ cells, isolated from both lymphoid tissue and peripheral blood, to inhibit HIV replication was compared. CD8+ cells from lymphoid tissue and peripheral blood of two HIV-infected long-term survivors suppressed HIV replication at a low CD8+:CD4+ cell ratio of 0.1. The CD8+ cells from the lymphoid tissue of a third asymptomatic subject suppressed HIV replication at a CD8+:CD4+ cell ratio of 0.25; the subject’s peripheral blood CD8+ cells showed this antiviral response at a lower ratio of 0.05. The lymphoid tissue CD8+ cells from two AIDS patients were not able to suppress HIV replication, and the peripheral blood CD8+ cells of only one of them suppressed HIV replication. The plasma viremia, cellular HIV load as well as the extent of pathology and virus expression in the lymphoid tissue of the two long-term survivors, were reduced compared with these parameters in the three other subjects. The data suggest that the extent of anti-HIV activity by CD8+ cells from lymphoid tissue relative to peripheral blood correlates best with the clinical state measured by lymphoid tissue pathology and HIV burden in lymphoid tissues and blood. The results add further emphasis to the importance of this cellular immune response in controlling HIV pathogenesis.
Analyses of the lymph nodes of human immunodeficiency virus (HIV)-infected individuals have revealed that these tissues are the main sites of HIV replication, even in patients who are clinically healthy (1–4). The lymph node is thus considered to be the reservoir of HIV in the body and perhaps the origin of HIV-induced pathogenesis in the host (5). Since HIV replication in the lymph node is never totally controlled, chronic immune stimulation can occur. Consequently, HIV may contribute to inducing pathogenesis in the lymphoid system by both direct virological and indirect immunological effects. This pathogenesis results in the functional compromise of the lymphoid system, which leads to opportunistic infections and the demise of the patient. However, compared with subjects with HIV-related disease, asymptomatic individuals who have been infected with HIV for many years have a significantly lower HIV burden in their peripheral blood, and their lymphoid tissues do not display extensive histopathology (6). Moreover, the replication of HIV in the lymph nodes is limited (7). Presumably a strong immune response by these subjects contributes to their viral and clinical latency.
To understand the mechanism conferring virus control and protection from HIV-mediated lymph node pathogenesis, we studied the CD8+ cell-mediated non-cytolytic suppression of HIV replication (8). This immune response has been investigated extensively by our laboratory and others (for reviews see refs. 9–11). This immune response by CD8+ cells of the peripheral blood has clinical relevance: a strong response correlates directly with a healthy clinical status (12) and a reduction in the response occurs concomitant with progression to disease (13). Further salient characteristics of this immune response are: (i) that it involves neither target cell lysis (10, 14, 15) nor modulation of activation of target CD4+ cells (8, 10); (ii) that it is independent of major histocompatibility complex restriction at the effector phase (12, 16); and (iii) that it is mediated in part by a novel soluble factor (8, 16–18), which does not share identity with any other known cytokines or chemokines (9).
We have evaluated the capacity of CD8+ cells from lymphoid tissue to suppress HIV replication. Five subjects were studied at different stages of HIV disease and the capacity of the CD8+ cells from the lymphoid and peripheral blood compartments to suppress HIV replication was compared. This study indicates that the lymphoid CD8+ cells can control HIV replication in vitro. Comparably high levels of non-cytolytic suppression of HIV replication by CD8+ cells from both the lymphoid tissues and peripheral blood correlated with a healthy clinical state. Reduced levels of lymphoid tissue CD8+ cell immune response either correlated with a poor clinical state or suggested that the clinical prognosis of the individual was poor. Thus, non-cytolytic suppression of HIV replication by lymphoid CD8+ cells may be important in maintaining the integrity of the lymphoid tissues and controlling HIV pathogenesis in vivo. Reduction in this antiviral response may presage development of disease. These studies add further emphasis to the importance of this cellular immune response in controlling HIV pathogenesis and emphasize the necessity for developing therapies that can enhance and maintain this anti-HIV activity.
MATERIALS AND METHODS
Subjects.
Five subjects whose clinical status represented different stages of HIV infection were studied. Two individuals were long-term survivors of HIV infection. Subject 1 has remained clinically asymptomatic since being diagnosed as HIV seropositive 10 years ago, although the estimated time of HIV infection is 12 years. Subject 2 also believes that he was infected with HIV 12 years ago and the HIV seropositive diagnosis was made 11 years ago. This subject has experienced some clinical symptoms including hairy leukoplakia, oral candidiasis, shingles, night sweats, and some weight loss. He was asymptomatic at the time of this study. Subject 3 believes that he was infected 6 years ago and has remained asymptomatic. The date of his HIV diagnosis was 1993 (2 years before removal of the lymph node). Subject 4, who was infected approximately 7 years ago, has had hairy leukoplakia and a marked CD4+ cell loss, but has not taken anti-retroviral therapy. Subject 5 was infected 3 years ago. He has experienced weight loss, a rapid CD4+ cell loss, development of Kaposi sarcoma, and has received comprehensive antiretroviral therapy. Lymphoid mononuclear cells were derived from lymphoid tissue removed with local anesthesia from all subjects except for subject 3, from whom tonsils were the source of lymphoid cells. The patients’ laboratory parameters are presented in Table 1, and their clinical category, according to the Centers for Disease Control and Prevention HIV classification (19), and CD4+ cell count are summarized in Table 2. This study received the approval of the Committee on Human Research, University of California (San Francisco).
Table 1.
Immunological and virological parameters of peripheral blood and lymphoid tissue
| Subject* | Cell subsets
|
Plasma virus load† | CD4+ cell virus load‡ % | Virus isolation§ | CD8+ cell anti-HIV activity¶ | ||
|---|---|---|---|---|---|---|---|
| CD4+ cells, % | CD8+ cells, % | CD4+:CD8+ cell ratio | |||||
| 1. Long-term survivor | |||||||
| Peripheral blood | 25 | 69 | 0.4 | 867 | 0.0003 | — | <0.05 |
| Lymph node | 48 | 44 | 1.1 | 0.0033 | — | <0.05 | |
| 2. Long-term survivor‖ | |||||||
| Peripheral blood | 35 | 43 | 0.8 | 4,554 | 0.0001 | — | <0.05 |
| Lymph node | 52 | 22 | 1.6 | 0.07 | — | 0.1 | |
| 3. Asymptomatic individual | |||||||
| Peripheral blood | 59 | 55 | 1.1 | 149,678 | 0.033 | — | <0.05 |
| Lymph node | 85 | 25 | 3.4 | 0.1 | + | 0.25 | |
| 4. AIDS patient | |||||||
| Peripheral blood | 8 | 91 | 0.1 | 398,994 | 0.1 | — | <0.1 |
| Lymph node | 6 | 81 | 0.1 | 0.01 | + | >1.0 | |
| 5. AIDS patient** | |||||||
| Peripheral blood | 13 | 80 | 0.2 | 55,573 | 0.0033 | + | 1.0 (81%) |
| Lymph node | ND | ND | 0.01 | ND | >0.5 | ||
ND, not determined.
See Table 2 for CD4+ cell count and Centers for Disease Control and Prevention HIV classification.
Expressed as HIV-1 RNA copies/ml, determined by RT–PCR.
Expressed by percentage as the least number of cells that released HIV as determined by infectious center assay.
Virus isolation by the A culture method (20) was performed by PHA stimulation of the subject’s PBMC and culture for 1 week before coculture with PHA-stimulated PBMC from an HIV-seronegative individual.
Expressed as the lowest CD8+:CD4+ cell ratio at which 90% or greater suppression of HIV replication occurred in autologous peripheral blood CD4+ cells; <, indicates that this was the lowest CD8+:CD4+ cell ratio tested; >, indicates that no anti-HIV activity was detected at this highest CD8+:CD4+ cell ratio tested.
The plasma sample for HIV RNA load determination was taken from this subject 3 weeks prior to lymph node removal.
Heterologous peripheral blood CD4+ cells were used to assay the anti-HIV activity of this patient’s CD8+ cells. The virus isolation was performed with PBMC from the previous visit to our laboratory, 3 months prior to removal of the lymph node.
Table 2.
A comparison of clinical state, lymphoid tissue pathology, and in situ hybridization studies of HIV RNA present in lymphoid tissues
| Subject | Clinical state* | Lymphoid tissue
|
|
|---|---|---|---|
| Pathology | HIV RNA in tissue by in situ hybridization† | ||
| 1 | 916 CD4+ cells/μl; HIV + 10 years; CDC A1 | Early FL, prominent 2° GC | Single GC positive, no individual cells with abundant HIV RNA (DNA positive) |
| 2 | 1078 CD4+ cells/μl; HIV + 11 years; CDC B1 | Early FL, prominent 2° GC | Two to three GC positive, several individual positive cells |
| 3 | 956 CD4+ cells/μl; HIV + 2 years; CDC A1 | FH, prominent 2° GC | Many GC positive, many individual positive cells |
| 4 | 213 CD4+ cells/μl; HIV + 7 years; CDC B3 | Late FL, no intact GC | FDC positive,‡ some individual positive cells |
| 5 | 87 CD4+ cells/μl; HIV + 3 years; CDC C3 | ND | ND |
FL, follicular lysis; GC, germinal centers; FH, follicular hyperplasia; ND, not determined; CDC, Centers for Disease Control and Prevention; FDC, follicular dendritic cells.
At the time of lymphoid tissue biopsy.
A positive GC result indicates that at least one site of HIV infection was detected.
Subject 4 lacked intact GC, but FDC-associated virus was evident in disintegrating GC. In situ hybridization methods are described in the text.
Mononuclear Cell Preparation.
Peripheral blood mononuclear cells (PBMC) were prepared by sodium diatrizoate density gradient centrifugation of heparinized blood according to previously described methods (20).
Lymphoid tissue mononuclear cells (LMC) were prepared by carefully teasing apart the lymphoid tissue using sterile forceps and gently rubbing the tissue over a sterile fine wire gauze. The lymphocytes were washed with Hanks’ balanced salt solution through the gauze, which retained residual lymphoid tissue. The lymphocytes were washed three times and used directly without further purification.
CD8+ and CD4+ Cell Separation.
CD8+ cells were obtained from freshly isolated mononuclear cells by immunomagnetic bead separation, with anti-CD8 antibody-coated immunomagnetic beads (Dynal, Lake Success, NY) (12). The CD8+ cells were >95% CD8+ as determined by flow cytometry (FACsort; Becton Dickinson). CD4+ cells were isolated from freshly prepared mononuclear cells of HIV-infected patients with anti-CD4 immunomagnetic beads (Dynal). T-cell analysis was performed by flow cytometry, according to previously published methods (12, 21).
Cell Culture.
The culture medium was RPMI 1640 (BioWhittaker) containing 10% heat inactivated (56°C, 30 min) fetal bovine serum (Gemini Biological Products, Calabasas, CA), 1% antibiotics (100 units/ml penicillin; 100 μg/ml streptomycin) and 2 mM glutamine. The medium was supplemented with either 10% natural interleukin 2 (Human T-stim; Collaborative Research), or human recombinant (r) interleukin 2 (Collaborative Research) at 200 units/ml, unless stated otherwise.
Assay of CD8+ Cell Anti-HIV Activity.
Non-cytolytic CD8+ cell anti-HIV activity was determined by the endogenous virus assay, as described (10). CD8+ cells and HIV-1 naturally infected CD4+ cells were cocultured in 24-well cell culture plates (Falcon, Becton Dickinson Labware) at multiple CD8+:CD4+ cell ratios, in duplicate where possible, and passed at 3-day intervals. The selected CD8+:CD4+ cell ratios ranged from 0.05 to 1:1. At the start of the assay, the cells were stimulated by phytohemagglutinin (PHA) (3 μg/ml; Sigma) for 3 days. In most experiments, CD8+ cells were cocultured with autologous CD4+ cells. However, in one AIDS patient, insufficient CD4+ cells were available and the cells were cocultured with heterologous naturally infected CD4+ cells from an HIV-1-infected donor. This choice of heterologous CD4+ cells was possible because this antiviral response is not major histocompatibility complex restricted at the effector stage (12). Previous studies have shown that the extent of the CD8+ cell anti-HIV activity is independent of whether the source of the naturally infected CD4+ target cells is either an asymptomatic or a symptomatic subject (12). Virus replication was quantitated by particle-associated reverse transcriptase (RT) activity (22), or in one case (subject 1), by p24 core antigen assay (Coulter). Inhibition of HIV replication was determined by comparing peak virus replication in wells containing CD4+ cells cultured alone with peak virus replication from wells containing CD4+ and CD8+ cell cocultures. Control of HIV replication was considered to have occurred if the RT level of the CD8+:CD4+ cell coculture was reduced by 90% or more. The extent of virus suppression was determined by the lowest CD8+:CD4+ cell ratio needed to control HIV replication.
HIV Detection and Quantification.
HIV isolation from cultured cells was performed according to previously published methods (20). The “A culture” method consisted of PHA stimulation (3 μg/ml) of the subject’s PBMC or LMC for 3 days and then culture for a further 4 days, before PHA-stimulated PBMC from HIV-seronegative individuals were added. The cultures were passaged every 3–4 days for a total of 30 days. HIV-1 was assayed by particle-associated RT activity for which a positive result is considered to be higher than 5000 counts per minute per ml of culture fluid. In this culture technique, the stimulated CD8+ cell subset typically prevents HIV release. Hence, the A culture can be an indirect indication of the extent of CD8+ cell antiviral activity.
Plasma HIV RNA quantitation was kindly performed by Cindy Christopherson in the laboratory of Shirley Kwok using polymerase chain reaction techniques described (23).
Infectious center assays were performed with subjects’ CD4+ cells positively selected from either PBMC or LMC with immunomagnetic beads. These cells were serially diluted 10-fold and each dilution of cells was inoculated onto 2 × 106 human PBMC. These PHA-stimulated PBMC were prepared from a healthy HIV-seronegative blood donor. The assay was performed in a 24-well plate in triplicate for each CD4+ cell dilution. Seven days later, an additional 106 PHA-stimulated PBMC were added to each culture. The cultures were passaged every 3–4 days for a total of 30 days. HIV-1 was assayed by particle-associated RT activity. In some cases, insufficient CD4+ cells were available for this assay and CD8+ cell-depleted mononuclear cells were used instead. The results are presented as the lowest percentage of the CD4+ cells plated, which induced RT activity in the target PBMC.
In situ hybridization studies to detect HIV RNA in lymphoid tissues were performed as previously described (7). This technique detects both HIV genomic RNA and HIV messenger RNA.
RESULTS
CD8+ Cell Anti-HIV Activity.
The anti-HIV activity of CD8+ cells from four subjects was assayed using autologous peripheral blood CD4+ cells. For subject 5, an AIDS patient with very few CD4+ cells, the antiviral activity of his CD8+ cells was assayed with heterologous CD4+ cells from an asymptomatic HIV-infected subject. The LMC CD8+ cells of two long-term survivors of HIV infection (subjects 1 and 2) had strong anti-HIV activity, that was comparable to the response by CD8+ cells from their peripheral blood. The CD8+ cells from both the LMC and the PBMC of subject 1 suppressed HIV replication at the lowest CD8+:CD4+ cell ratio tested (0.05; Table 1). The CD8+ cells from both sites from the second long-term survivor, subject 2, showed similar levels of antiviral activity, except that the LMC CD8+ cells required a slightly higher CD8+:CD4+ cell ratio (0.1 versus <0.05; Table 1) to control HIV replication. The lymphoid CD8+ cells of the third subject, an asymptomatic individual for 6 years (subject 3), showed antiviral activity at the CD8+:CD4+ cell ratio of 0.25. Thus, the anti-HIV response was reduced compared with that shown by CD8+ cells from his peripheral blood (CD8+:CD4+ cell ratio = <0.05; Table 1).
The LMC CD8+ cells of the AIDS patient, subject 4 (213 CD4+ cells/μl of blood), failed to show antiviral activity even at the highest CD8+:CD4+ cell ratio tested, 1.0 (Table 1). However, his peripheral blood CD8+ cells showed strong control of HIV replication at the lowest CD8+:CD4+ cell ratio tested, 0.1. Finally, the CD8+ cells from the LMCs of the second AIDS patient, subject 5 (87 CD4+ cells/μl of blood), failed to show antiviral activity (Table 1) and even enhanced HIV replication (data not shown). His peripheral blood CD8+ cells inhibited HIV replication by only 81% at a CD8+:CD4+ cell ratio of 1.0 and lower ratios also enhanced HIV replication (data not shown).
The antiviral activity of CD8+ cells from the three healthy subjects was also assayed using autologous CD4+ cells from the lymphoid tissue. The results were consistent with those of Table 1 (data not shown). The three subjects’ CD8+ cells showed strong control of HIV replication, despite the elevated viral load of the lymphoid CD4+ cells relative to that of the PBMC CD4+ cells (Table 1). Furthermore, the same pattern of slightly reduced antiviral activity by the lymphoid CD8+ cells compared with the PBMC CD8+ cells was demonstrated for subjects 2 and 3. Insufficient LMC were available for this comparison to be made for the two symptomatic patients.
HIV isolation by the A culture method provides an indirect indication of CD8+ cell anti-HIV activity (see Materials and Methods). Culture of both the LMC and PBMC from the long-term survivors, subjects 1 and 2, by this method did not yield virus replication (Table 1). The LMC A culture of the asymptomatic individual, subject 3, did release virus (Table 1), consistent with the reduced anti-HIV activity of this individual’s LMC CD8+ cells (Table 1). However, no HIV replication occurred in the A culture of his PBMC.
HIV replication by the A culture method was achieved more readily with cells of the symptomatic subjects than cells from the three healthy individuals. The A culture of the LMC from the AIDS patient, subject 4, released HIV, whereas his PBMC A culture did not (Table 1). Due to insufficient cells, A cultures were not performed with the mononuclear cells from the other AIDS patient, subject 5, at the time of lymph node biopsy. However, at his previous visit to our laboratory (3 months earlier) HIV was readily released from his PBMC A culture (Table 1).
Plasma and Cellular Viral Load.
The quantity of HIV RNA in the subjects’ plasma was determined by RT–PCR (Table 1). The lowest amounts of plasma HIV-1 RNA from the five subjects in this study were found in the long-term survivors of HIV infection, subject 1 (867 RNA copies/ml), and subject 2 (4554 RNA copies/ml, Table 1). The asymptomatic individual, subject 3, had a relatively high plasma virus load (149,678 RNA copies/ml, Table 1), but the highest virus load was found in the AIDS patient, subject 4 (398,994 RNA copies/ml, Table 1). The virus load of the second AIDS patient, subject 5 (55,573 RNA copies/ml, Table 1) was lower than that of either subject 3 or 4. This virus load was also lower than expected for the low level of CD8+ cell antiviral activity, but presumably this result reflects a combination of extensive antiretroviral therapy and a low CD4+ cell count.
The cellular viral loads in the lymphoid and peripheral blood compartments were determined by infectious center assay (Table 1). The viral burden of the lymphoid tissue of each of the three healthy subjects was higher than that of the peripheral blood (≈1–3 log10). The virus level of the AIDS patient, subject 4, was 10-fold higher in the peripheral blood compared with the lymphoid tissue, whereas in the second AIDS patient, subject 5, it was 3-fold higher in the lymphoid tissue.
HIV RNA Expression in Lymphoid Tissues.
In situ hybridization studies were performed to detect and localize HIV RNA in the lymphoid tissues of the subjects studied (Fig. 1). A comparison with the pathology of the lymphoid tissues was made (Table 2). The lymph node of the long-term survivor, subject 1, showed the lowest level of viral RNA expression of all the lymphoid tissue studied (Fig. 1A). One signal was detected over a germinal center confirming the presence of HIV in this lymph node and supporting the infectious center data (Table 1). However, no individual cells with abundant viral RNA were detected. It is noteworthy that in situ DNA PCR did detect HIV-infected cells within the germinal center, which were evidently not producing HIV RNA (data not shown). The lymph node of the second long-term survivor, subject 2, compared with that of subject 1, showed elevated levels of viral RNA in virions trapped by FDC. Viral RNA was detected in two to three germinal centers and individual HIV RNA positive cells were identified (Fig. 1B). The tonsil tissue of subject 3 revealed HIV trapped in the FDC of many germinal centers and many individual cells within the germinal centers contained HIV RNA (Fig. 1C). The subject 4 lymphoid tissue showed late stage follicular lysis consistent with progression of HIV-related disease. Although intact germinal centers were not evident in this tissue, in situ hybridization suggested that HIV was trapped by residual FDCs and occasional cells within the germinal centers contained HIV RNA (Fig. 1D). The lymphoid tissue from subject 5 was not sufficient for conducting these RNA analyses.
Figure 1.
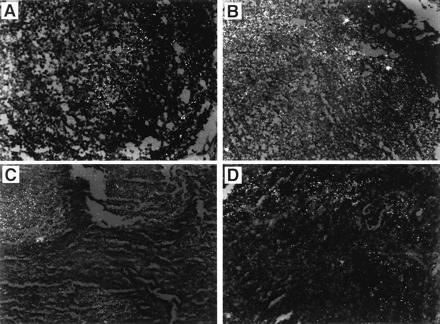
In situ hybridization to detect HIV RNA in lymphoid tissues. (A) The lymph node of the long-term survivor, subject 1, showing a single HIV RNA signal in the germinal center. (B) The lymph node of the long-term survivor, subject 2, showing individual HIV RNA positive cells. (C) The tonsil tissue of subject 3 reveals many germinal centers harboring several individual cells containing HIV RNA. (D) The lymphoid tissue of subject 4 (which showed late-stage follicular lysis consistent with progression of HIV-related disease) shows HIV trapped by residual FDCs and some cells within the germinal centers containing HIV RNA.
DISCUSSION
This study indicates that CD8+ cells from the lymphoid tissue of healthy HIV-infected individuals, similar to these cells in the blood, provide substantial control of HIV replication in vitro. Furthermore, this noncytolytic suppression of HIV replication by lymphoid CD8+ cells, in comparison to CD8+ cells from PBMC, appears to correlate best with the clinical status. In these studies, clinical status is defined by the criteria of clinical symptoms, HIV RNA plasma copy number, and HIV transcriptional activity in lymphoid tissue cells. The point prevalence studies in the present report suggest that as the capacity of the lymphoid CD8+ cells to control HIV replication weakens, the clinical status of the patient declines.
The correlation between lymphoid CD8+ cell anti-HIV activity and clinical status is reflected in the anti-HIV response of the CD8+ cells of subjects 3, 4 and also subject 2, and the extent of virus expression in their cells (Table 1). The LMC CD8+ cells of subject 3, an asymptomatic individual who has been HIV-infected for 6 years, demonstrated reduced antiviral activity relative to his PBMC CD8+ cells. His LMC CD8+ cells showed antiviral activity at the CD8+:CD4+ cell ratio of 0.25, but this level was reduced compared with that shown by his peripheral blood CD8+ cells (CD8+:CD4+ cell ratio = <0.05; Table 1). Although this subject lacks clinical symptoms of chronic HIV infection, his HIV RNA plasma copy number is high (149,678 RNA copies/ml) relative to the levels of the two long-term survivors of HIV infection in this study (subject 1, 867 RNA copies/ml; subject 2, 4,554 RNA copies/ml), which is suggestive of a poorer clinical prognosis (24). Furthermore, HIV-infected cells of the lymphoid tissue of subject 3 showed the highest level of HIV transcriptional activity of the three subjects studied (subjects 1–3) with intact germinal centers (Fig. 1, Table 2). These data suggest a strong correlation between reduced LMC CD8+ cell antiviral activity and clinical deterioration. This issue is being investigated as the subject is followed over time.
The results observed with subject 3 could also reflect the source of his lymphoid CD8+ cells, the tonsils, but the data are consistent with both the HIV RNA plasma copy number, virus isolation studies, and cellular virus load in this subject. Furthermore, on the occasion of his tonsilectomy and a follow-up visit 8 months later, the peripheral blood CD8+ cells of this still asymptomatic subject have shown low levels of anti-HIV activity when tested with heterologous CD4+ cells acutely infected with HIV (data not shown). This finding is consistent with a poor clinical prognosis (12, 13).
The lymphoid CD8+ cells of subject 4 failed to show antiviral activity even at the highest CD8+:CD4+ cell ratio tested, 1.0 (Table 1). However, his peripheral blood CD8+ cells showed strong control of HIV replication at the lowest CD8+:CD4+ cell ratio tested, 0.1. Consistent with the undetectable LMC CD8+ cell anti-HIV activity and late-stage follicular lysis, the HIV RNA plasma copy number of this subject was the highest of the five studied (398,994 RNA copies/ml). Moreover, virus was readily isolated from his PBMC (A culture) and his cellular virus load was substantial (Table 1).
The anti-HIV response of the LMC CD8+ cells of subject 2 was also reduced compared with that of his peripheral blood CD8+ cells and this subject has experienced some clinical symptoms of HIV infection. Although slight, this difference was observed as well when the LMC and PBMC CD8+ cells of this subject were tested against autologous LMC CD4+ cells (data not shown). In summary, the data from subjects 2, 3, and 4 suggest that a reduced LMC CD8+ cell anti-HIV activity relative to that of PBMC CD8+ cells correlates closely with either the clinical status of the subject or suggests a poor clinical prognosis.
As cited above for the subjects 2, 3, and 4, the extent of the antiviral activity of the CD8+ cells from all subjects in this study was supported by the virus isolation assay (see Materials and Methods). The absence of HIV replication in the A culture technique reflects CD8+ cell control of autologous HIV at in vivo effector:target cell ratios. The A cultures of both the LMC and PBMC of the two long-term survivors of HIV infection, subjects 1 and 2, did not release infectious virus. These data are consistent with the strong antiviral activity of the CD8+ cells from both cellular sites of these two subjects. As noted above, the LMC A cultures of subjects 3 and 4, but not their PBMC A cultures, released virus. These data are also consistent with the relative level of the CD8+ cell antiviral activity in the LMC compared with the PBMC of both subjects.
The results of the in situ hybridization studies are also consistent with the hypothesis that decreased CD8+ cell anti-HIV activity in vitro reflects loss of control of HIV replication in vivo. When strong CD8+ cell anti-HIV activity was observed, the number of cells in the lymphoid tissue expressing HIV RNA was reduced (Fig. 1) as was the extent of HIV-related pathogenesis in the lymphoid tissues (Table 2). Furthermore, while the lymphoid tissues of subjects 1, 2, and 3 are almost indistinguishable by pathology, CD8+ cell antiviral activity could be predictive of a decline in the clinical status of subject 2, and perhaps of subject 3. Subject 1 has remained clinically asymptomatic, but subject 2 has experienced some clinical symptoms of HIV disease (see Materials and Methods); we are monitoring subject 3.
The in vitro studies of the present report suggest one possible mechanism (CD8+ cell response) by which HIV can be controlled at the lymphoid site where cells exist in an activated state and are particularly susceptible to HIV infection (25, 26). The number of subjects of the present report is limited due to the difficulty in obtaining lymphoid tissues. However, the CD4+ cell counts and thus the clinical state of the individuals studied represent the wide variations observed with HIV-infected people. Therefore, the observations on CD8+ cell activities of these subjects should be representative but need to be confirmed in future studies with larger numbers of patients. Nevertheless, a clear trend is represented by these data: a reduction in the capacity of lymphoid CD8+ cells to suppress HIV replication correlates with a compromised clinical state and an increased viral burden, both in the lymphoid tissue and the peripheral blood. These studies with lymphoid tissue support previous findings that strong CD8+ cell suppression of HIV replication could play a major role in preventing the HIV pathogenic process (12, 13). These observations place further emphasis on therapeutic directions for strengthening this cellular immune response (27).
Acknowledgments
We thank Bineetha Ramachandran, Roland Orque, and Tesi Low for technical assistance, Sue Fujimura for flow cytometry studies, and Kris Gebhard for in situ hybridization studies. The tonsilectomy was performed by Dr. K. H. Wu (Department of Head and Neck Surgery, Kaiser Hospital, San Francisco). Christine Beglinger helped in the preparation of this manuscript. D.J.B. was supported in part by the University of California Universitywide AIDS Research Program (Grant F94-SF-008).
Footnotes
Abbreviations: PBMC, peripheral blood mononuclear cells; LMC, lymphoid tissue mononuclear cells; PHA, phytohemagglutinin; RT, reverse transcriptase; FDC, follicular dendritic cells.
References
- 1.Embretson J, Zupancic M, Ribas J L, Burke A, Racz P, Tenner-Racz K, Haase A T. Nature (London) 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 2.Fox C H, Tenner-Racz K, Racz P, Firpo A, Pizzo P A, Fauci A S. J Infect Dis. 1991;164:1051–1057. doi: 10.1093/infdis/164.6.1051. [DOI] [PubMed] [Google Scholar]
- 3.Pantaleo G, Graziosi C, Butini L, Pizzo P A, Schnittman S M, Kotler D P, Fauci A S. Proc Natl Acad Sci USA. 1991;88:9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. Nature (London) 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 5.Pantaleo G, Cohen R J, Schwartzentruber D J, Graziosi C, Vaccarezza M, Fauci A S. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;10:S6–S14. [PubMed] [Google Scholar]
- 6.Pantaleo G, Menzo S, Vaccarazza M, Graziosi C, Cohen O J, Demarest J F, Montefiori D, Orenstein J M, Fox C, Schrager L K, Margolick J B, Buchbinder S, Giorgi J V, Fauci A S. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 7.Embretson J, Zupancic M, Beneke J, Till M, Wolinsky S, Ribas J L, Burke A, Haase A T. Proc Natl Acad Sci USA. 1993;90:357–361. doi: 10.1073/pnas.90.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker C M, Moody D J, Stites D P, Levy J A. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 9.Levy J A, Mackewicz C E, Barker E. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 10.Mackewicz C, Levy J A. AIDS Res Hum Retroviruses. 1992;8:1039–1050. doi: 10.1089/aid.1992.8.1039. [DOI] [PubMed] [Google Scholar]
- 11.Blackbourn D J, Mackewicz C, Barker E, Levy J A. Res Immunol. 1994;145:653–658. doi: 10.1016/s0923-2494(05)80049-5. [DOI] [PubMed] [Google Scholar]
- 12.Mackewicz C E, Ortega H W, Levy J A. J Clin Invest. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landay A L, Mackewicz C, Levy J A. Clin Immunol Immunopathol. 1993;69:106–116. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 14.Wiviott L D, Walker C M, Levy J A. Cell Immunol. 1990;128:628–634. doi: 10.1016/0008-8749(90)90054-u. [DOI] [PubMed] [Google Scholar]
- 15.Walker C M, Erikson A L, Hsueh F C, Levy J A. J Virol. 1991;65:5921–5927. doi: 10.1128/jvi.65.11.5921-5927.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinchmann J E, Gaudernack G, Vartdal F. J Immunol. 1990;144:2961–2966. [PubMed] [Google Scholar]
- 17.Mackewicz C E, Ortega H, Levy J A. Cell Immunol. 1994;153:329–343. doi: 10.1006/cimm.1994.1032. [DOI] [PubMed] [Google Scholar]
- 18.Mackewicz C E, Blackbourn D J, Levy J A. Proc Natl Acad Sci USA. 1995;92:2308–2312. doi: 10.1073/pnas.92.6.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control. Morb Mortal Wkly Rep. 1993;41:1–19. [Google Scholar]
- 20.Castro B A, Weiss C D, Wiviott L D, Levy J A. J Clin Microbiol. 1988;26:2371–2376. doi: 10.1128/jcm.26.11.2371-2376.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy J A, Tobler L H, McHugh T M, Casavant C H, Stites D P. Clin Immunol Immunopathol. 1985;35:328–336. doi: 10.1016/0090-1229(85)90093-5. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman A D, Banapour B, Levy J A. Virology. 1985;147:326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- 23.Mulder J, McKinney N, Christopherson C, Sninksy J, Greenfield L, Kwok S. J Clin Microbiol. 1994;32:292–300. doi: 10.1128/jcm.32.2.292-300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellors J W, Rinaldo C R, Jr, Gupta P, White R M, Todd J A, Kingsley L A. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 25.Tang S, Patterson B, Levy J A. J Virol. 1995;69:5659–5665. doi: 10.1128/jvi.69.9.5659-5665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weissman D, Barker T D, Fauci A S. J Exp Med. 1996;183:687–692. doi: 10.1084/jem.183.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker E, Mackewicz C E, Levy J A. Proc Natl Acad Sci USA. 1995;92:11135–11139. doi: 10.1073/pnas.92.24.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]


