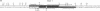Abstract
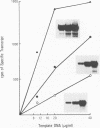
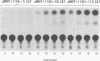
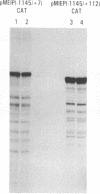
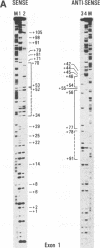
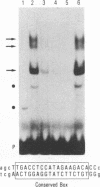
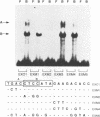
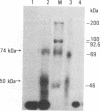
The major immediate-early promoter (MIEP) of human cytomegalovirus is a remarkably strong RNA polymerase II transcription control unit. We have identified and characterized a novel regulatory domain associated with MIEP downstream from the initiation site of transcription. The downstream regulatory region was first identified by analyzing a series of mutations in the 5' untranslated leader exon. This regulatory domain was shown to enhance the number of functional initiation complexes without significantly altering the apparent elongation rate by RNA polymerase II transcription. In addition, run-off in vitro transcription and DNA-binding experiments identified two distinct downstream elements that specify the interaction of cellular transcription factors. One of these elements contains a reiterated sequence motif, present twice within the leader exon. The second element is an 18-bp sequence located at approximately nucleotide position +33 that is conserved between strains of cytomegalovirus from different species. On the basis of two criteria, an oligonucleotide competition assay and oligomerization upstream of the promoter, the binding of factors to the conserved box was shown to be critical for mediating the level of transcription from MIEP. Two discrete cellular nuclear proteins, designated LTF A and B (for leader transcription factor A and B binding factors), were found to specifically recognize the conserved element. This study of promoter-proximal elements within transcribed sequences demonstrates the recognition of the control domain at the DNA level that functions to increase the number of committed RNA polymerase II transcription complexes.
Full text
PDF

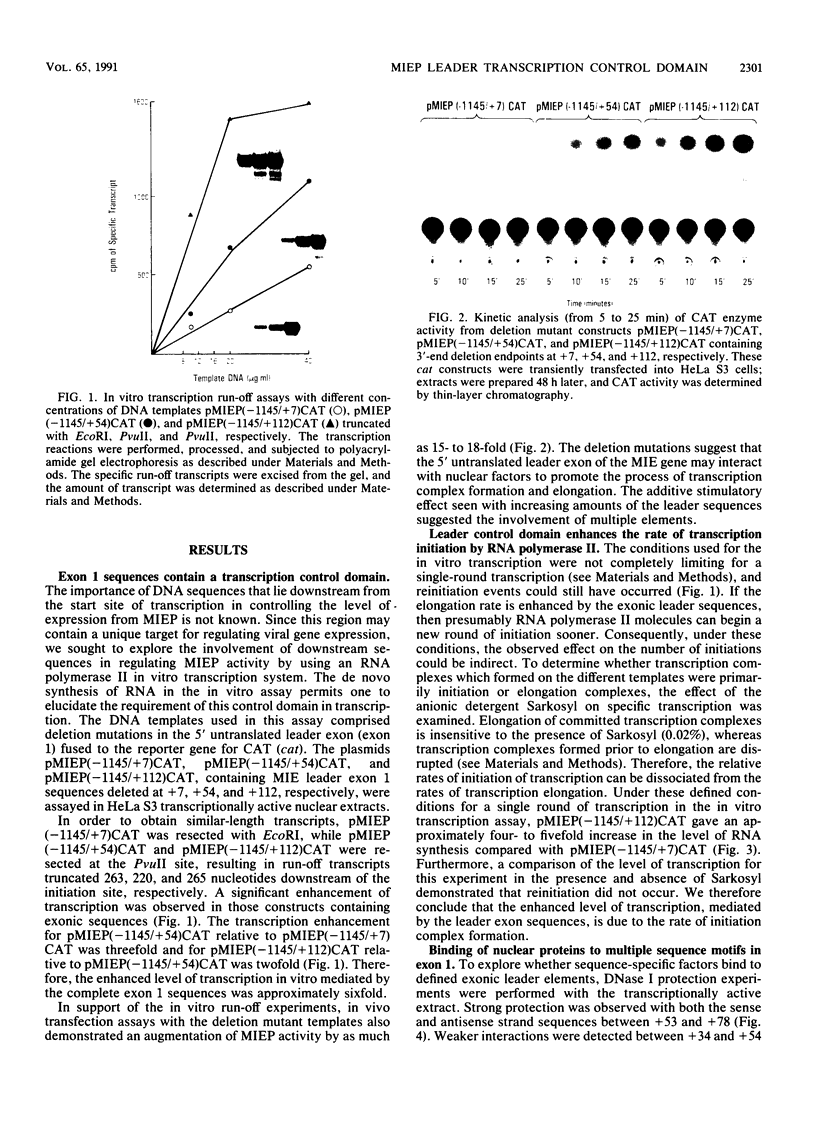


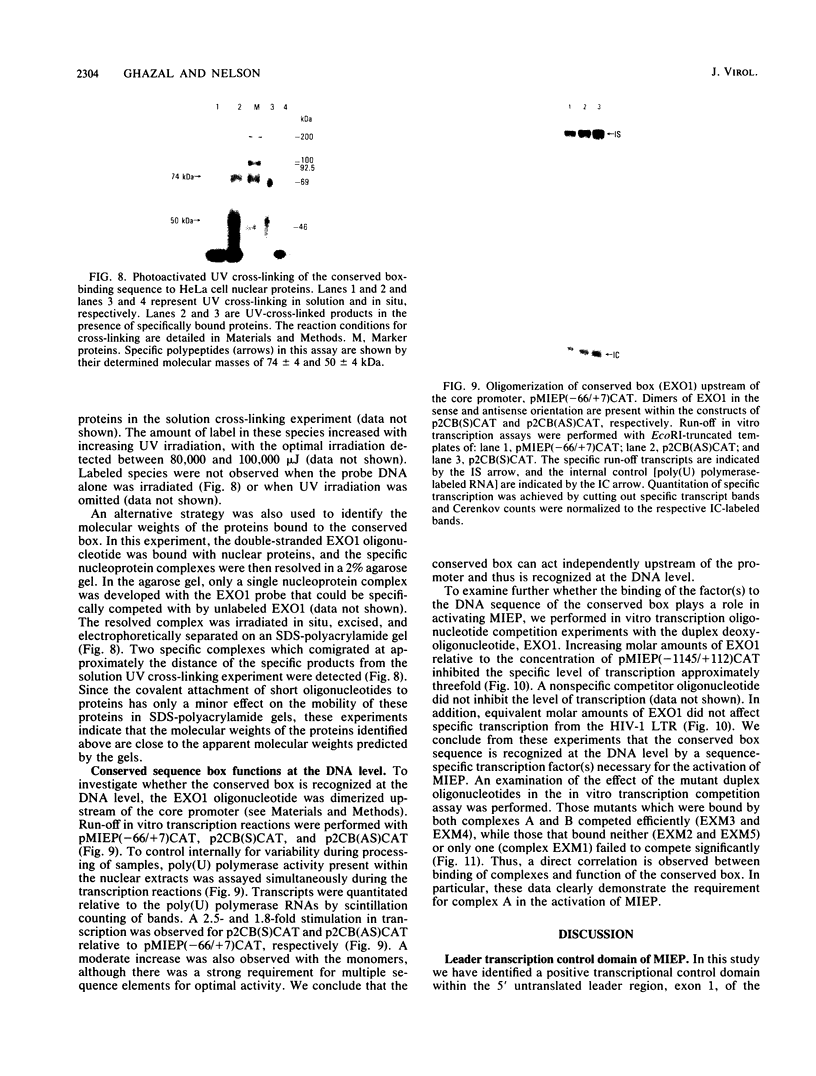
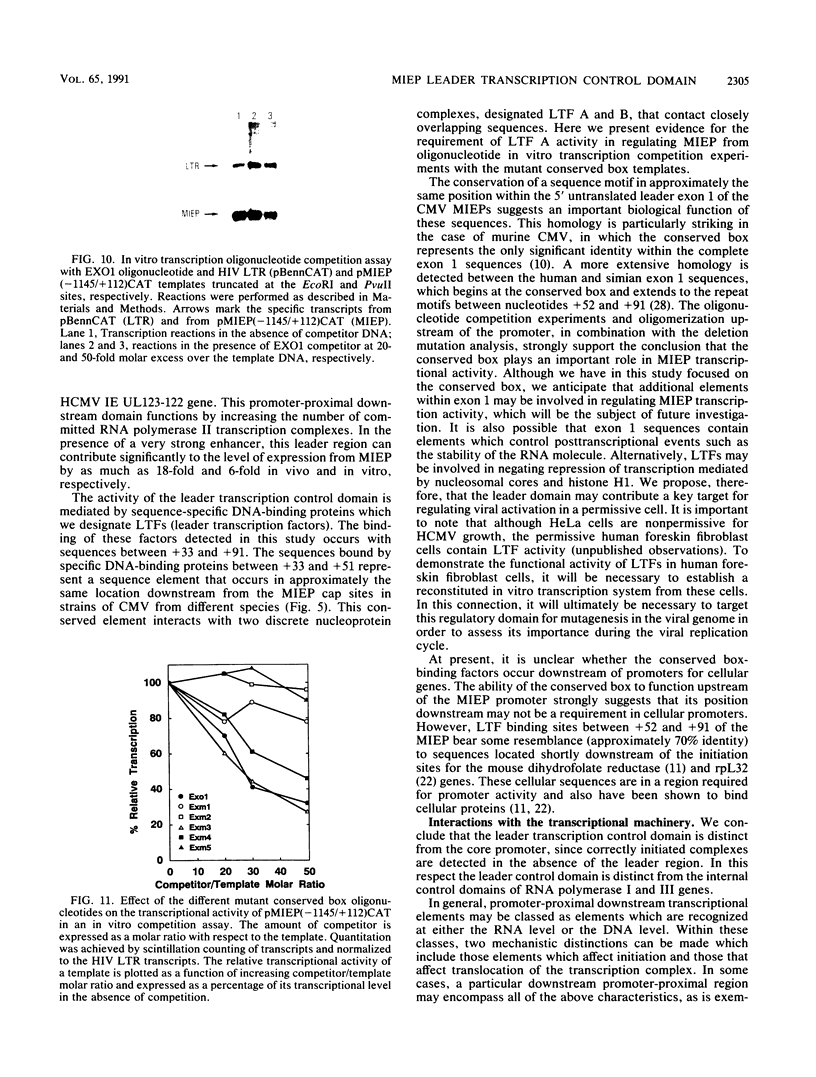


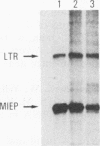
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayer D. E., Dynan W. S. A downstream-element-binding factor facilitates assembly of a functional preinitiation complex at the simian virus 40 major late promoter. Mol Cell Biol. 1990 Jul;10(7):3635–3645. doi: 10.1128/mcb.10.7.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B., Gatignol A., Rabson A. B., Jeang K. T. TAR-independent activation of the HIV-1 LTR: evidence that tat requires specific regions of the promoter. Cell. 1990 Aug 24;62(4):757–767. doi: 10.1016/0092-8674(90)90120-4. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Braddock M., Chambers A., Wilson W., Esnouf M. P., Adams S. E., Kingsman A. J., Kingsman S. M. HIV-1 TAT "activates" presynthesized RNA in the nucleus. Cell. 1989 Jul 28;58(2):269–279. doi: 10.1016/0092-8674(89)90841-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang Y. N., Crawford S., Stall J., Rawlins D. R., Jeang K. T., Hayward G. S. The palindromic series I repeats in the simian cytomegalovirus major immediate-early promoter behave as both strong basal enhancers and cyclic AMP response elements. J Virol. 1990 Jan;64(1):264–277. doi: 10.1128/jvi.64.1.264-277.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Derse D., Casey J. W. Two elements in the bovine leukemia virus long terminal repeat that regulate gene expression. Science. 1986 Mar 21;231(4744):1437–1440. doi: 10.1126/science.3006241. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Keil G. M., Weber F., Jasin M., Schaffner W., Koszinowski U. H. A long and complex enhancer activates transcription of the gene coding for the highly abundant immediate early mRNA in murine cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8325–8329. doi: 10.1073/pnas.82.24.8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnham P. J., Means A. L. Sequences downstream of the transcription initiation site modulate the activity of the murine dihydrofolate reductase promoter. Mol Cell Biol. 1990 Apr;10(4):1390–1398. doi: 10.1128/mcb.10.4.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. A., Harrich D., Soultanakis E., Wu F., Mitsuyasu R., Gaynor R. B. Human immunodeficiency virus type 1 LTR TATA and TAR region sequences required for transcriptional regulation. EMBO J. 1989 Mar;8(3):765–778. doi: 10.1002/j.1460-2075.1989.tb03437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. A., Wu F. K., Mitsuyasu R., Gaynor R. B. Interactions of cellular proteins involved in the transcriptional regulation of the human immunodeficiency virus. EMBO J. 1987 Dec 1;6(12):3761–3770. doi: 10.1002/j.1460-2075.1987.tb02711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P. A rapid and selective method for generating deletions, insertions and clustered point mutations. Biotechniques. 1989 Nov-Dec;7(10):1070–1076. [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Fleckenstein B., Hennighausen L. Binding of transcription factors and creation of a large nucleoprotein complex on the human cytomegalovirus enhancer. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3658–3662. doi: 10.1073/pnas.84.11.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Hennighausen L. Multiple sequence-specific transcription factors modulate cytomegalovirus enhancer activity in vitro. Mol Cell Biol. 1988 Apr;8(4):1809–1811. doi: 10.1128/mcb.8.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Hennighausen L. Specific interactions between transcription factors and the promoter-regulatory region of the human cytomegalovirus major immediate-early gene. J Virol. 1988 Mar;62(3):1076–1079. doi: 10.1128/jvi.62.3.1076-1079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Reynolds-Kohler C., Hennighausen L., Nelson J. A. Interactions between cellular regulatory proteins and a unique sequence region in the human cytomegalovirus major immediate-early promoter. Virology. 1990 Jan;174(1):18–25. doi: 10.1016/0042-6822(90)90049-w. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Equipotent mouse ribosomal protein promoters have a similar architecture that includes internal sequence elements. Genes Dev. 1989 Nov;3(11):1789–1800. doi: 10.1101/gad.3.11.1789. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Separation and partial characterization of three functional steps in transcription initiation by human RNA polymerase II. J Biol Chem. 1985 Jul 5;260(13):8163–8172. [PubMed] [Google Scholar]
- Hennighausen L., Fleckenstein B. Nuclear factor 1 interacts with five DNA elements in the promoter region of the human cytomegalovirus major immediate early gene. EMBO J. 1986 Jun;5(6):1367–1371. doi: 10.1002/j.1460-2075.1986.tb04368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Monick M. M., Liu B., Stinski M. F. The promoter-regulatory region of the major immediate-early gene of human cytomegalovirus responds to T-lymphocyte stimulation and contains functional cyclic AMP-response elements. J Virol. 1989 Jul;63(7):3026–3033. doi: 10.1128/jvi.63.7.3026-3033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits A., Smith D. H., Jakobovits E. B., Capon D. J. A discrete element 3' of human immunodeficiency virus 1 (HIV-1) and HIV-2 mRNA initiation sites mediates transcriptional activation by an HIV trans activator. Mol Cell Biol. 1988 Jun;8(6):2555–2561. doi: 10.1128/mcb.8.6.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Rawlins D. R., Rosenfeld P. J., Shero J. H., Kelly T. J., Hayward G. S. Multiple tandemly repeated binding sites for cellular nuclear factor 1 that surround the major immediate-early promoters of simian and human cytomegalovirus. J Virol. 1987 May;61(5):1559–1570. doi: 10.1128/jvi.61.5.1559-1570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Luciw P. A., Duchange N. Structural arrangements of transcription control domains within the 5'-untranslated leader regions of the HIV-1 and HIV-2 promoters. Genes Dev. 1988 Sep;2(9):1101–1114. doi: 10.1101/gad.2.9.1101. [DOI] [PubMed] [Google Scholar]
- Kao S. Y., Calman A. F., Luciw P. A., Peterlin B. M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987 Dec 3;330(6147):489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Laspia M. F., Rice A. P., Mathews M. B. HIV-1 Tat protein increases transcriptional initiation and stabilizes elongation. Cell. 1989 Oct 20;59(2):283–292. doi: 10.1016/0092-8674(89)90290-0. [DOI] [PubMed] [Google Scholar]
- Leong K., Lee W., Berk A. J. High-level transcription from the adenovirus major late promoter requires downstream binding sites for late-phase-specific factors. J Virol. 1990 Jan;64(1):51–60. doi: 10.1128/jvi.64.1.51-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubon H., Ghazal P., Hennighausen L., Reynolds-Kohler C., Lockshin C., Nelson J. Cell-specific activity of the modulator region in the human cytomegalovirus major immediate-early gene. Mol Cell Biol. 1989 Mar;9(3):1342–1345. doi: 10.1128/mcb.9.3.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubon H., Ghazal P., Nelson J. A., Hennighausen L. Cell-specific activity of the human immunodeficiency virus enhancer repeat in vitro. AIDS Res Hum Retroviruses. 1988 Oct;4(5):381–391. doi: 10.1089/aid.1988.4.381. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Grodzicker T., Tjian R. Downstream sequences affect transcription initiation from the adenovirus major late promoter. Mol Cell Biol. 1986 Jul;6(7):2684–2694. doi: 10.1128/mcb.6.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak R. A., Calnan B. J., Frankel A. D., Sharp P. A. HIV-1 Tat protein trans-activates transcription in vitro. Cell. 1990 Nov 16;63(4):791–802. doi: 10.1016/0092-8674(90)90145-5. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Gnann J. W., Jr, Ghazal P. Regulation and tissue-specific expression of human cytomegalovirus. Curr Top Microbiol Immunol. 1990;154:75–100. doi: 10.1007/978-3-642-74980-3_4. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Groudine M. Transcriptional regulation of the human cytomegalovirus major immediate-early gene is associated with induction of DNase I-hypersensitive sites. Mol Cell Biol. 1986 Feb;6(2):452–461. doi: 10.1128/mcb.6.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A., Reynolds-Kohler C., Smith B. A. Negative and positive regulation by a short segment in the 5'-flanking region of the human cytomegalovirus major immediate-early gene. Mol Cell Biol. 1987 Nov;7(11):4125–4129. doi: 10.1128/mcb.7.11.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikovits W., Jr, Mar J. H., Ordahl C. P. Muscle-specific activity of the skeletal troponin I promoter requires interaction between upstream regulatory sequences and elements contained within the first transcribed exon. Mol Cell Biol. 1990 Jul;10(7):3468–3482. doi: 10.1128/mcb.10.7.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niller H. H., Hennighausen L. Phytohemagglutinin-induced activity of cyclic AMP (cAMP) response elements from cytomegalovirus is reduced by cyclosporine and synergistically enhanced by cAMP. J Virol. 1990 May;64(5):2388–2391. doi: 10.1128/jvi.64.5.2388-2391.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin B. M., Luciw P. A., Barr P. J., Walker M. D. Elevated levels of mRNA can account for the trans-activation of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9734–9738. doi: 10.1073/pnas.83.24.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. P., Mathews M. B. Transcriptional but not translational regulation of HIV-1 by the tat gene product. Nature. 1988 Apr 7;332(6164):551–553. doi: 10.1038/332551a0. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Roy S., Parkin N. T., Rosen C., Itovitch J., Sonenberg N. Structural requirements for trans activation of human immunodeficiency virus type 1 long terminal repeat-directed gene expression by tat: importance of base pairing, loop sequence, and bulges in the tat-responsive sequence. J Virol. 1990 Mar;64(3):1402–1406. doi: 10.1128/jvi.64.3.1402-1406.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambucetti L. C., Cherrington J. M., Wilkinson G. W., Mocarski E. S. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989 Dec 20;8(13):4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby M. J., Peterlin B. M. Trans-activation by HIV-1 Tat via a heterologous RNA binding protein. Cell. 1990 Aug 24;62(4):769–776. doi: 10.1016/0092-8674(90)90121-t. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Marciniak R. A. HIV TAR: an RNA enhancer? Cell. 1989 Oct 20;59(2):229–230. doi: 10.1016/0092-8674(89)90279-1. [DOI] [PubMed] [Google Scholar]
- Shelbourn S. L., Kothari S. K., Sissons J. G., Sinclair J. H. Repression of human cytomegalovirus gene expression associated with a novel immediate early regulatory region binding factor. Nucleic Acids Res. 1989 Nov 25;17(22):9165–9171. doi: 10.1093/nar/17.22.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate C., Zapp M. L., Green M. R. Activation of transcription by HIV-1 Tat protein tethered to nascent RNA through another protein. Nature. 1990 Jun 14;345(6276):640–642. doi: 10.1038/345640a0. [DOI] [PubMed] [Google Scholar]
- Stamminger T., Fickenscher H., Fleckenstein B. Cell type-specific induction of the major immediate early enhancer of human cytomegalovirus by cyclic AMP. J Gen Virol. 1990 Jan;71(Pt 1):105–113. doi: 10.1099/0022-1317-71-1-105. [DOI] [PubMed] [Google Scholar]
- Stinski M. F., Roehr T. J. Activation of the major immediate early gene of human cytomegalovirus by cis-acting elements in the promoter-regulatory sequence and by virus-specific trans-acting components. J Virol. 1985 Aug;55(2):431–441. doi: 10.1128/jvi.55.2.431-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theill L. E., Wiborg O., Vuust J. Cell-specific expression of the human gastrin gene: evidence for a control element located downstream of the TATA box. Mol Cell Biol. 1987 Dec;7(12):4329–4336. doi: 10.1128/mcb.7.12.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stenberg R. M., Goins W. F., Stinski M. F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toohey M. G., Jones K. A. In vitro formation of short RNA polymerase II transcripts that terminate within the HIV-1 and HIV-2 promoter-proximal downstream regions. Genes Dev. 1989 Mar;3(3):265–282. doi: 10.1101/gad.3.3.265. [DOI] [PubMed] [Google Scholar]
- Wondisford F. E., Farr E. A., Radovick S., Steinfelder H. J., Moates J. M., McClaskey J. H., Weintraub B. D. Thyroid hormone inhibition of human thyrotropin beta-subunit gene expression is mediated by a cis-acting element located in the first exon. J Biol Chem. 1989 Sep 5;264(25):14601–14604. [PubMed] [Google Scholar]
- Yang J. Q., Remmers E. F., Marcu K. B. The first exon of the c-myc proto-oncogene contains a novel positive control element. EMBO J. 1986 Dec 20;5(13):3553–3562. doi: 10.1002/j.1460-2075.1986.tb04682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]