Abstract
Patients with mild Alzheimer’s disease (AD) display a greater tendency to endorse unstudied items as ‘old’ on memory tests than healthy older adults. This liberal response bias may result in mistaken beliefs about the completion of common tasks. This research attempted to determine whether it was possible to shift the response bias of mild AD patients to be more conservative on a recognition memory test through behavioral intervention. Patients with mild AD and matched controls were evaluated with two almost identical paradigms, separated by about one week. For each session, 30 words were studied and 60 words (half studied, half novel) were shown at test. During one session participants were told that 30% of words were old, and at the other session that 70% were old. We found that both groups were able to shift their response bias between the two conditions. That patients with mild AD were able to successfully shift their response bias demonstrates that—despite their overall liberal response bias and poor memory relative to controls—one component of metamemorial ability is preserved in patients with mild AD.
Keywords: memory, Alzheimer’ s disease, response bias, metamemory, discrimination
Alzheimer’s disease (AD) causes progressive memory impairment leading to lesser than normal levels of true recognition and greater than normal levels of false recognition (for review see Budson, Wolk, Chong, & Waring, 2006). Discrimination in recognition memory tests is often calculated by using a “corrected score” of hits minus false alarms. However, this corrected score can oversimplify the interpretation of memory performance. By failing to consider hits and false alarms independently, important features of AD patients’ memory impairments may be obscured. Further understanding of the causes of false memories is necessary to aid in improving this condition.
Discrimination is one important factor in memory performance. In the context of a recognition memory task, discrimination is expressed by the ability to distinguish items previously seen from those which were not seen during the prior study portion. Another factor impacting memory is response bias, which is the overall tendency to respond “old” to test items. Response bias may be liberal (responding “old” greater than 50% of the time) or conservative (responding “old” less than 50% of the time) (Snodgrass & Corwin, 1988; Macmillan & Creelman, 2005; Huh, Kramer, Gazzaley, & Delis, 2006). Although perfect discrimination is always accompanied by a neutral bias, as discrimination decreases the range of possible biases increases. Individuals with high rates of false recognition show an abnormally liberal bias; understanding this liberal bias may be important in understanding false memories.
Several studies have explored the impact of aging upon response bias in healthy older adults. Marquie and Baracat (2000) found that older adults show poorer recognition discrimination than younger adults, but found no overall effect of sex or age upon response bias, except at the highest levels of education. There was an effect of age found only in the most educated group, where the older participants used a more conservative criterion for responding. Huh et al. (2006) found that increasing age correlated with an increasingly liberal response bias in older adults (aged 75–89), but not in a younger (aged 35–49) age cohort.
Impaired discrimination in AD patients has been well documented, but there have been fewer studies exploring response bias in AD. In one such study, Balota, Burgess, Cortese, and Adams (2002) found patients with mild AD demonstrated a more liberal response bias to high frequency words than to low frequency words. In another study, Snodgrass and Corwin (1988) found that AD patients showed worse discrimination and an abnormally liberal response bias compared to controls. When comparing memory performance between patients with AD and patients with amnesia due to mixed etiologies, they noted a difference marked by an abnormally liberal response bias found only in AD patients, although poor discrimination was found in both patient groups. Other studies exploring discrimination and response bias in amnesics of mixed etiologies showed that despite low discrimination, they still demonstrate normal or even conservative bias (Koutstaal, Verfaellie & Schacter, 2001; Schacter, Verfaellie, Anes & Racine, 1998). Closer examination of Korsakoff’s amnesia patients showed slightly higher false recognition rates than those with amnesia due to mixed etiologies (Schacter et al., 1998). The authors hypothesized that these results may be due to additional damage to frontal networks present only in Korsakoff’s amnesia patients. Bartok et al. (1997) also noticed that response bias was more liberal in AD patients than controls and bias did not correspond with disease severity, as measured by the MMSE. They considered the possibility that bias is dependent upon a process other than memory, possibly the ability to monitor previous responses.
In our previous investigation of response bias we compared patients with mild AD and older controls on study-test levels of increasing lengths. We found that the mild AD patients’ bias was more liberal than controls, and that increasing the memory load by increasing the number of words at study and test affected only discrimination, and not response bias. Even after matching levels of discrimination between the groups, the mild AD patients still showed an abnormally liberal response bias (Budson et al., 2006). This liberal response bias is one important factor leading to false memories for items that were actually unstudied.
There have been few studies attempting to deliberately manipulate response bias, and debate continues regarding whether it is possible to manipulate criterion level item by item or only between word lists. Wixted and Stretch (2000, based upon data from Stretch & Wixted, 1998) concluded that while they found no support for item by item criterion changes due to item strength within a list, young adult participants could apply metamemorial knowledge to shift criterion between lists. Wixted and Stretch (2000) hypothesized that although participants are generally unwilling to put forth the mental effort needed to shift criterion level this frequently, it is theoretically possible to do so on an item by item basis given the necessary effort.
Despite the conclusions of Wixted and Stretch (2000) that it is unlikely that criterion would shift frequently within a recognition memory test, others found that with explicit feedback young adults could dynamically shift their bias within the trial blocks (Rhodes & Jacoby, 2007). Miller, Handy, Cutler, Inati, and Wolford (2001) were also able to manipulate discrimination and response bias independently in young controls in an fMRI study of recognition memory. During the study session, half of the words were shown once and half were shown thrice. At test, half of the words were presented in red and half were presented in green. Item by item, subjects were instructed to be very liberal in responding “yes” for the green words, and to be very conservative for the red words. The authors found that activations associated with changes in bias were located in lateral cerebellum, lateral parietal lobe, and dorsolateral prefrontal cortex, whereas activations associated with changes in discrimination were located in anterior and medial prefrontal cortex. Reber and Squire (1999) found that experimental instruction to relax response decision criterion did not affect discrimination in their study of amnesics and controls. Verfaellie, Giovanello and Keane (2001, expt. 2) succeeded in shifting the response bias of amnesics and controls by giving instructions that either 30% or 70% of items on a recognition test were old, while in actuality 50% of the items were old in both conditions. They also demonstrated that although there was no impact of condition upon level of discrimination in controls, amnesics actually increased their discrimination in the 70% condition.
The ability to adjust one’s response criteria based upon external considerations may be demonstration of metamemorial ability, which is one form of memory awareness. Metamemory is often used to mean the ability to reflect upon one’s memory performance. Some models of metamemory include components of monitoring and control, where monitoring refers to a collection of inputs about one’s knowledge and performance, and control refers to self-regulation of behavior (Nelson & Narens, 1990). A third model combines these two processes into a feedback system where monitoring affects control and vice versa by communicating information back and forth (Nelson, 1996). This feedback system is necessary to achieve proficient memory functioning. Introspection derived from feedback is vital in a memory system, as even an imperfect system containing errors and distortions uses introspection to modify behavior (Nelson, 1996).
The results of investigations into metamemorial ability in AD may be examined in consideration of the distinctions between retrospective monitoring and prospective monitoring ability, and of global predictions. Item-by-item metamemory evaluation using prospective measures, such as judgment-of-learning (JOL) and feeling-of-knowing (FOK) have shown differing results in AD patients from retrospective measures such as judgment-of-confidence (JOC), which are different still from reports of global predictions (Moulin, Perfect & Jones, 2000b; Nelson & Narens, 1990).
Past research has shown mixed results about whether AD patients have impaired metamemorial ability. Some researchers have found AD patients are overconfident in assessing their memory abilities and show a lack of awareness of their memory loss (Kaszniak & Zak, 1996, McGlynn & Kaszniak, 1991). Backman and Lipinska (1993) found memory monitoring from FOK and JOC to be intact in AD patients, despite deficits in fact retrieval. They hypothesized that although memory is impaired early in the course of the disease, metamemory may be spared until more severe stages. Based upon results of a JOL task, Moulin, Perfect, and Jones (2000a) suggested that recall may not live up to the expectations of AD patients, but there is no significant metacognitive deficit at encoding. Others concluded that AD patients’ FOK judgments are more impaired in episodic memory tasks than semantic memory tasks (Souchay, Isingrini & Gil, 2002). A metaanalysis by Pannu and Kaszniak (2005) also pointed out that evidence of intact metamemory in AD patients was presented in tasks requiring semantic memory, while episodic memory tasks showed differences in metamemory performance. Budson, Dodson, Daffner and Schacter (2005) found that although AD patients’ ability to use the metamemorial strategy of the distinctiveness heuristic was intact, they had difficulty correctly applying this heuristic due to their primary episodic memory deficit.
Assessment of global predictions made by AD patients, where prospective and retrospective judgments are compared, have shown that although they may overestimate their accuracy in both instances, AD patients may still be monitoring memory performance by using feedback regarding performance (Moulin, Perfect & Jones, 2000b). Their shifts between pre-study and post-study predictions for future memory accuracy demonstrate sensitivity to monitoring processes. Although the memory monitoring by AD patients may be poorly calibrated, it nonetheless reflects that they are able to utilize feedback from an encoding task. In short, the literature clearly shows that—rather than a broad degradation of all metamemorial abilities—AD patients have impairments of some aspects of metamemory and preservation of others.
We hoped to further understand the relationship between metamemory, response bias, and discrimination in AD by attempting to manipulate response bias in mild to very mild AD patients. Modeling after the methods of Verfaellie et al. (2001), we administered a recognition memory paradigm in which during one session participants were told that 30% of test words were old, and at the other session that 70% were old—when in actuality 50% of the words were old in both sessions. Using this paradigm we were interested in answering two questions. First, is one aspect of metamemory in AD patients preserved to the extent that they are able to use instructions to moderate their response bias to become more conservative? Using such interventions in AD patients could be very useful as a way to self-monitor and control memory performance and possibly reduce their false recognition. Second, we wanted to determine whether discrimination of the AD patients would improve if their bias became more liberal, as occurred in amnesics. We predicted that, as in amnesics, it would be possible to shift response bias to be more or less lenient in AD patients by using verbal instructions to create a structure for varying decision-making criterion between two otherwise equivalent conditions. Given that even mild AD patients already show a liberal bias at baseline, however, we thought it would be unlikely that their discrimination would improve with relaxed response criteria in the 70%condition relative to the 30% condition.
Methods
Participant
The study sample included eleven patients with a clinical diagnosis of probable AD (NINCDS-ADRDA criteria; McKhann, Drachman, Folstein, Katzman, & Price, 1984) who were recruited from the Memory Disorders Unit, Brigham and Women’s Hospital, and the Boston University Alzheimer’s Disease Center, both in Boston, MA, USA. These patients were each assessed and diagnosed by neurologists, and were otherwise healthy. The neuropsychological tests used to assess patients’ clinical diagnosis included the MMSE (Folstein, Folstein, & McHugh, 1975), the Dementia Scale (Blessed, Tomlinson, and Roth, 1968), CERAD Word List Memory Test (Morris et al., 1989), Verbal Fluency to letters and categories (Monsch, Bondi, Butters, Salmon, Katzman, & Thal, 1992), the Frontal Assessment Battery (Dubois, Slachevsky, Litvan, & Pillom, 2000), the Geriatric Depression Scale (Koenig, Meador, Cohen, & Blazer, 1988), and the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983). A control group consisted of eleven healthy community-dwelling older adults recruited from the Boston University Alzheimer’s Disease Center, from spouses of patients, and by the use of flyers posted in community centers. Written informed consent was obtained from all participants. The Human Subjects Committees of Brigham and Women’s Hospital and the Edith Nourse Rogers Memorial Veterans Hospital, Bedford, MA approved this study. Participants were paid $10 per hour for their participation. Patients with AD were in the very mild or mild stage of the disease based upon their performance on the MMSE (mean = 25.1, range 21–28). Details of some of the participants’ neuropsychological test performance can be found in Table 1. Participants were screened for clinically significant depression, alcohol or drug abuse, past stroke or traumatic brain damage. All participants had normal or corrected to normal vision. The control group was matched to the patients on the basis of age (patient mean =77.8 years, range =65–84 years; older adult mean = 77.1 years, range = 67–84 years), and education (patient mean =16.1 years, range = 12–20 years; older adult mean = 16.1 years, range = 12–19 years). There were 6 female patients and 6 female older adult controls.
Table 1.
Results of the neuropsychological tests for patients with AD and healthy older adult controls
| AD Patient | Control | df | F | P | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| Test | |||||
| Global cognition score | |||||
| MMSE (Folstein et al., 1975) | 25.09 (2.21) | 29.36 (.92) | 1,20 | 34.95 | <.001 |
| Verbal fluency (Monsch et al., 1992) | |||||
| Letters (FAS) | 28.89 (11.16) | 42.10 (12.41) | 1,17 | 5.9 | 0.027 |
| Categories (animals, fruits, vegetables) | 19.89 (6.75) | 48.40 (13.62) | 1,17 | 32.19 | <.001 |
| Frontal lobe function | |||||
| Frontal Assessment Battery (Dubois et al., 2000) | 14.50 (2.43) | 17.00 (1.29) | 1,11 | 5.62 | .037 |
| Memory (CERAD; Morris et al., 1989) | |||||
| Word List Memory | 9.64 (3.44) | 19.60 (3.66) | 1,19 | 41.35 | <.001 |
| Word List Recall | 1.55 (1.69) | 6.70 (1.77) | 1,19 | 46.53 | <.001 |
| Word List Recognition | 6.55 (2.70) | 9.70 (0.48) | 1,19 | 13.24 | 0.002 |
Values for df, F, and P are from one-way ANOVAs between AD patients and controls.
Verbal fluency was not available for 2 patients, the frontal assessment battery was not available for 5 patients and 4 controls, and the CERAD was not available for 1 control.
Materials
132 words were selected from the University of Western Australia MRC Psycholinguistic Database (http://www.psy.uwa.edu/au/MRCDataBase/uwa_mrc.htm). The words were selected using the parameters of Kucera-Francis written frequency of 20–45, word length of 4–7 letters, familiarity rating of 389–587, and age of acquisition rating (AOA) of 285–525. Parameters for familiarity rating and AOA were chosen by selecting +/−1 SD from the mean as the minimum and maximum values. Words that were vulgar, foreign, or highly similar to other words were removed, and the final 120 words used in the experiment were selected randomly from the remaining words. The words were then counterbalanced into 4 lists of 30 words each. ANOVAs were used to assure equivalent AOA, familiarity, Kucera-Francis frequency, and word length between lists. Eight study-test levels were constructed by varying list presentation order and test instructions. The experiment was programmed using PsyScope software (Cohen, MacWhinney, Flatt, & Provost, 1993) on an Apple G4 iBook. Words were shown in black font on a white background for 2000 ms with an interstimulus interval of 400 ms. Test words were presented until a response was entered, with the next word immediately following the previous response.
Procedure
Participants were evaluated on two study-test sessions with a median delay of 7 days (M=9.24, range 6–23, excluding one control participant who did not return for 5 months due to illness). At each session they were instructed to read aloud and try to remember 30 words for a subsequent test. Participants were then given a 10-minute delay in which they were asked to complete a simple number puzzle. This was followed by a 60-word recognition memory test, where participants were told to respond “old” to) words they had studied, and “new” to words they did not remember studying. Half of all items were previously studied and half were novel. In the 30% condition, participants were told that 3 out of every 10 words were from the studied list, so 30% of items were “old”. At the 70% condition, they were told 7 out of every 10 words were previously seen, so 70%were “old”1. A card with this information was placed at the top of the computer screen during the test phase. In an effort to reduce working memory load for AD patients, the card was left visible throughout the test phase. Sessions were counterbalanced so half of participants experienced the 30% condition on the first session, and half experienced the 70% condition first.
Results
Standard neuropsychological tests
AD patients performed significantly worse than older controls on all standard neuropsychological tests given, as evidenced in Table 1.
Hits and false alarms
Comparison between groups across the two conditions using a repeated measures ANOVA with group (AD patients versus older adults) as a between subjects factor and condition (30% versus 70%) and item type (hits versus false alarms) as within-subjects factors showed main effects of condition (F(1,20)=33.41, p<.0005, η2=.63) and item type (F(1,20)=216.42, p<.0005, η2=.92), but not group (F(1,20)=1.80, p=.195, η2=.08). There was an interaction present between group × item type (F(1,20)=51.27, p<.0005, η2=.72), but not group × condition (F(1,20)<1.0, η2<.01), condition × item type (F(1,20) <1.0, η2<.01), or condition × item type × group (F(1,20)<1.0, η2=.04). The effect of condition is present because participants had more hits and false alarms at the 70% condition than the 30% condition, suggesting that our manipulation was successful. The effect of item type is present because overall participants made more hits than false alarms. Additional analyses were performed to better understand the interactions between item type and condition or group.
Comparison between groups and conditions with repeated measures ANOVA for hits demonstrated an effect of condition (F(1,20)=11.76, p<.005, η2=.37) and an effect of group (F(1,20)=7.10, p=.015, η2=.26), but no interaction between condition × group (F(1,20)<1.0, η2=.04). These effects indicate participants endorsed more items at the 70% condition overall as being ‘old’, and the AD patients had fewer hits than controls in both conditions, as seen in Table 2.
Table 2.
Proportion of hits and false alarms in patients with AD and older adults
| Hits |
False Alarms |
|||
|---|---|---|---|---|
| 30% cond | 70% cond | 30% cond | 70% cond | |
| AD (SD) | 0.62 (0.22) | 0.75 (0.13) | 0.42 (0.15) | 0.52 (0.16) |
| Older Adult (SD) | 0.78 (0.11) | 0.86 (0.06) | 0.13 (0.10) | 0.27 (0.20) |
Conducting the same analysis for false alarms revealed an effect of condition (F(1,20)=15.60, p=.001, η2=.44) and also of group (F(1,20)=20.81, p<.0005, η2=.51), and again no interaction was present (F(1,20) <1.0, η2=.02). The effect of condition is present because both participant groups made more false alarms in the 70% condition than the 30% condition. An effect of group is present because the AD patients had a higher number of false alarms than the older controls across conditions.
d’ and C
To calculate discrimination and response bias, the measures d’ and C were used, respectively, which are calculated from formulas from signal detection theory (Snodgrass & Corwin, 1988; Macmillan & Creelman, 2005)2. Higher values for d’ indicate greater recognition accuracy, zero indicates chance performance, and negative values indicate that a participant endorsed more novel items as ‘old’ than studied items. Response bias is a measure of a participant’s tendency to respond “old” or “new” to items on a recognition memory task. Bias score of zero indicates a neutral bias; in this case one is equally likely to endorse an item as being old or new. Perfect discrimination is always accompanied by a neutral bias. Positive values for C mean a conservative response bias is present; endorsing less than half of items seen at test as “old”. Negative values for C signify a liberal response bias; responding “old” more than half of the time.
d’ data
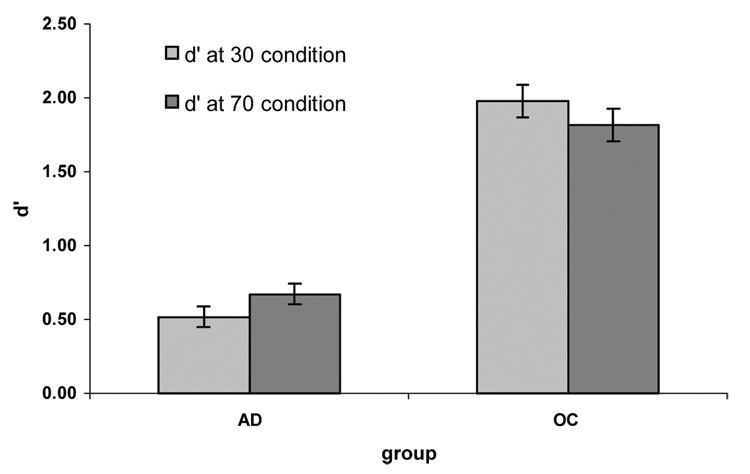
We performed a repeated measures ANOVA for discrimination with group (older controls versus patients) as a between subjects factor, and condition (30% versus 70%) as a within subjects factor. We found a main effect of group (F(1,20)=36.26, p<.0005, η2=.65) and no effect of condition (F(1,20)<.01, η2<.01). There was also no interaction between group × condition (F(1,20)=1.46, p=.241, η2=.07). The effect of group shows that the AD patients had significantly lower discrimination than the older controls, whereas the lack of effect of condition or interaction with condition shows that discrimination did not differ across conditions for either group.
C data
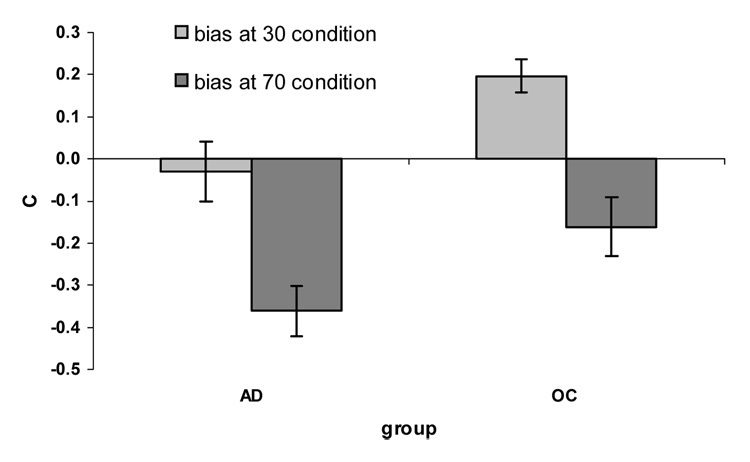
We performed a repeated measures ANOVA for bias with group (older controls versus patients) as a between subjects factor, and condition (30% versus 70%) as a within subjects factor. We found a main effect of condition (F(1,20)=34.45, p<.0005, η2=.63), but no effect of group (F(1,20)=1.87, p=.187, η2=.09). There was no interaction of group × condition (F(1,20) <0.1, η2<.01). The effect of condition is present because both groups had a significantly more liberal response bias at the 70% condition compared to the 30% condition (OC t(10)=4.34, p=.001, AD t(10)=3.97, p=.003).
In order to assure that our results were not due solely to an inability by one group to adjust response bias across conditions, bias at the 70% condition was subtracted from bias at the 30% condition and this value was compared between groups. A one-way ANOVA for ability to shift bias found there was no significant difference between groups (F(1,20) <0.1). The response bias of older adults changed from conservative at the 30% condition (M = .20, SD=.26) to slightly liberal at 70% condition (M = −.16, SD=.42). Response bias of patients with AD changed from slightly liberal at the 30% condition (M = −.03, SD=.47) to more liberal at 70% condition (M = −.36, SD=.38). It is interesting to note that the average bias of AD patients at 30% condition was more conservative than older adults at 70% condition.
To assure our critical result was not affected by test condition order, we used a repeated measures ANOVA with group (older controls versus patients) and order of levels (30% first versus 70% first) as between subjects factors, and condition (30% versus 70%) as within subjects factors. There was no effect of level order (F(1,18)=.10, η2<.01). There was no interaction between group and level order (F(1,18)<1.0, η2=.04) or condition and level order (F(1,18)<0.10, η2<01), or condition × group × level order (F(1,18)<1.0, η2<.01).
Discussion
Our purpose in conducting this study was to explore whether AD patients could use external behavioral interventions to moderate their response bias. Our results support our first hypothesis: mild AD patients were able to use external information to shift to a more conservative response bias. Our second hypothesis was also supported: although mild AD patients were able to shift their response bias, their discrimination was not improved.
Our 30% and 70% manipulations at test successfully shifted the response bias of both groups to become relatively more conservative and more liberal, respectively. AD patients showed significantly lower discrimination than the older control group. Shifting response criteria did not impact discrimination for either group. Unlike the results from amnesic patients, encouraging mild AD patients to relax response criterion further did not improve their ability to retrieve information from episodic memory. It is worth noting, however, that the response bias of the AD patients in the 30% condition was more conservative than the older control group in the 70% condition, showing that it is possible for the AD patients to shift their bias to a level within the range of normally aging adults. It must, nevertheless, be acknowledged that even when the AD patients were told that only 30% of items were previously seen, they still endorsed over half of the items as having been studied. Having reviewed our results, we now turn to a discussion of how our findings may be informative regarding (1) our understanding of metamemory in mild AD, (2) the neurophysiology of response bias, and (3) methods of improving the lives of patients with AD.
With respect to existing models of metamemory, our data show that in early stages of AD, the specific metamemorial ability to use external cues to shift response bias is preserved, even in episodic memory tasks. The feedback system between monitoring by using the external behavioral cue and control as regulation of responses inform one another in order allow AD patients to appropriately utilize this information to influence their responding. As in Moulin et al. (2000b), despite the poor calibration evidenced when AD patients still endorsed over half the items as ‘old’ in the 30% condition, metamemory was intact well enough for them to be able to shift response bias successfully between the 30% and 70% conditions. Regardless of AD patients’ response bias being poorly calibrated towards an excessively liberal criterion, at least this one feature of metamemorial ability is intact in early stages of the disease.
Previously we found that AD patients’ liberal response bias was not simply attributable to their impaired discrimination (Budson et al., 2006). In the present study we found dissociation between mild AD patients’ impaired discrimination and intact ability to shift response bias. Several previous studies in healthy and memory-impaired populations have lent support to the idea that discrimination and response bias rely upon different neuroanatomical structures. Snodgrass and Corwin (1988) found that patients with amnesia due to mixed etiologies showed very impaired discrimination despite a normal response bias. Kramer et al. (2005) provided an assessment of the volume of brain structures in patients with a variety of dementias and concluded that the best and only predictor of delayed recall ability was hippocampal volume. On the other hand, frontal lobe volumes were a significant predictor of response bias, with smaller volumes leading to more liberal biases displayed. Supporting the idea of the frontal lobes being important in response bias, patients with frontal lobe lesions have demonstrated elevated false alarms, but hit rates comparable to controls (Budson et al., 2002). Swick and Knight (1999) found that patients with frontal lobe lesions showed impairments in the use of strategies and source monitoring, and they exhibited a more liberal response bias compared to controls.
The theory that discrimination and response bias are localized in separate areas of the brain has also been supported in recent years by studies with both fMRI and ERP technologies. Using ERPs, Windmann, Urbach and Kutas (2002) concluded that response bias is related to a top-down control process mediated by prefrontal cortex, which may be important for the setting of a criterion level. The dorsolateral and anterior frontal cortices (DLFC and AFC, respectively) in particular may play a large role in the regulation of response bias. In a meta-analysis, Fletcher & Henson (2001) concluded that the DLFC may be used in selection and monitoring of information in working memory, as well as a second-stage episodic retrieval. Additionally, they considered that AFC may operate high-level control processes such as changing strategies of intentional retrieval for goal-directed behavior. As mentioned above, Miller et al. (2001) found that brain activation associated with changes in bias were located in lateral cerebellum, lateral parietal lobe, and DLFC, whereas activations associated with changes in discrimination were located in AFC and medial prefrontal cortex. In summary, these studies suggest that aspects of metacognition related to response bias and its manipulation may depend upon the frontal lobes, and also possibly upon frontal lobe interaction with lateral parietal cortex.
Considering the critical role of the frontal cortex for metacognitive shifts in response bias, we speculate that the relative preservation of frontal lobe function in our patients with very mild and mild AD is the likely explanation for their preserved metamemorial ability needed to shift their response bias using behavioral interventions. This is not to say frontal lobe function is normal in patients with very mild AD (our patients were impaired on the frontal assessment battery, Dubois et al., 2000), only that frontal lobe function is relatively spared compared to many other cognitive abilities. Supporting this idea is the AD patients’ performance on word fluency to letters versus categories relative to controls. Word fluency to letters has been shown to be more sensitive to frontal lobe function than word fluency to categories (Lezak, 1995). Although the AD patients showed impairment on both word fluency to letters and to categories, there was a striking disparity between their performance and that of controls on fluency for letters (13 word difference) versus categories (29 word difference). A repeated-measures ANOVA comparing letter and category fluency in AD patients and controls confirms this relative difference, finding not only an overall effect of group (F(1,17)=18.26, p=.001, η2=.52), but also a group × test interaction (F(1,17)=16.25, p=.001, η2=.49).
In future experimental designs we can investigate whether mild AD patients have awareness of their responding patterns by using a post-test verbal self-report. Another consideration for future investigations is to examine the effect of the response instruction manipulation presented in one recognition test paradigm upon subsequent recognition tests presented without the instruction manipulation. Both of these additional explorations may yield further knowledge about response monitoring and metamemory in mild AD.
The results of this study have clinical implications for techniques that may allow AD patients in early disease states to reduce false memories by shifting their response criterion to normal levels. Applying a stricter criterion may be helpful when considering whether important aspects of daily life have been performed. The patients’ baseline liberal response bias creates a default response of “yes, I must have done it” or “yes, I must have seen it” with reference to experiences that they are unsure have occurred. Whereas patients may be inclined to say, “Yes, I think I did it,” it would be beneficial to more conservatively say, “I’m not sure, so I’ll check.” Despite poor memory accuracy for performing tasks, encouraging patients with mild AD to use metamemorial monitoring and control to reconsider and recheck their actions may reduce the occurrence of negative outcomes from false memories. External behavioral cues suggesting one should be more conservative when unsure could be applied to scenarios such as checking whether doors were locked, medications taken, or the stovetop turned off. Sessions with a cognitive occupational therapist or social worker may also be helpful in attempting to shift response criterion to be more conservative in general. Such manipulations may be useful in aiding patients in their daily lives.
Figure 1.
Discrimination by group and condition.
Figure 2.
Shift in response bias by group and condition.
Acknowledgements
This research was supported by National Institute on Aging grants R01 AG025815 and P30 AG13846. We thank Brandon Ally for reading earlier versions of this manuscript, and also Ellen Beth for her help with this project.
Footnotes
One perspective of our experiment’s procedure is that we manipulated features somewhat similar to demand characteristics, while holding the actual paradigm constant.
Values for d’ and C were converted using the formulas from Snodgrass and Corwin (1988) because they are undefined when proportion of responses equals 0 or 1. H = (# hits + 0.5)/(# studied items + 1); FA = (#false alarms + 0.5)/(# unstudied items + 1).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backman L, Lipinska B. Monitoring of general knowledge: Evidence for preservation in early Alzheimer’s disease. Neuropsychologia. 1993;31:335–345. doi: 10.1016/0028-3932(93)90157-u. [DOI] [PubMed] [Google Scholar]
- Balota DA, Burgess GC, Cortese MJ, Adams DR. The word-frequency mirror effect in young, old, and early-stage Alzheimer's disease: Evidence for two processes in episodic recognition performance. Journal of Memory and Language. 2002;46:199–226. [Google Scholar]
- Bartok JA, Wilson CS, Giordani B, Keys BA, Persad CC, Foster NL, et al. Varying patterns of verbal recall, recognition, and response bias with progression of Alzheimer’s disease. Aging Neuropsychology and Cognition. 1997;4:266–272. doi: 10.1080/13825589708256651. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Budson AE, Sullivan AL, Mayer E, Daffner KR, Black PM, Schacter DL. Suppression of false recognition in Alzheimer’s disease and in patients with frontal lobe lesions. Brain. 2002;125:2750–2765. doi: 10.1093/brain/awf277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Dodson CS, Daffner KR, Schacter DL. Metacognition and false recognition in Alzheimer’s disease: Further exploration of the distinctiveness heuristic. Neuropsychology. 2005;19:253–258. doi: 10.1037/0894-4105.19.2.253. [DOI] [PubMed] [Google Scholar]
- Budson AE, Wolk DA, Chong H, Waring JD. Episodic memory in Alzheimer’s disease: Separating response bias from discrimination. Neuropsychologia. 2006;44:2222–2232. doi: 10.1016/j.neuropsychologia.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: A frontal assessment battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Memory and the frontal lobes: the fMRI evidence. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Huh TJ, Kramer JH, Gazzaley A, Delis DC. Response Bias and aging on a recognition memory task. Journal of the International Neuropsychological Society. 2006;12:1–7. doi: 10.1017/S1355617706060024. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kaszniak AW, Zak MG. On the neuropsychology of metamemory: Contributions from the study of amnesia and dementia. Learning and Individual Differences. 1996;8:355–381. [Google Scholar]
- Koenig HG, Meador KG, Cohen HJ, Blazer DG. Self-rated depression scales and screening for major depression in the older hospitalized patient with medical illness. Journal of the American Geriatrics Society. 1988;36:699–706. doi: 10.1111/j.1532-5415.1988.tb07171.x. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Verfaellie M, Schacter DL. Recognizing identical versus similar categorically related common objects: further evidence for degraded gist representations in amnesia. Neuropsychology. 2001;15:268–289. [PubMed] [Google Scholar]
- Kramer JH, Rosen HJ, Du AT, Schuff N, Hollnagel C, Weiner MW, et al. Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology. 2005;19:799–805. doi: 10.1037/0894-4105.19.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A user’s guide. New Jersey: Lawrence Erlbaum Associates; 2005. [Google Scholar]
- Marquie JC, Baracat B. Effects of age, education, and sex on response bias in a recognition task. Journal of Gerontology: Psychological Sciences. 2000;55:266–272. doi: 10.1093/geronb/55.5.p266. [DOI] [PubMed] [Google Scholar]
- McGlynn SM, Kaszniak AW. Unawareness of deficits in dementia and schizophrenia. In: Priatano GP, Schacter DL, editors. Awareness of deficit after brain injury: Theoretical and clinical aspects. New York: Oxford University Press.; 1991. pp. 84–110. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miller MB, Handy TC, Cutler J, Inati S, Wolford GL. Brain activations associated with shifts in response criterion on a recognition test. Canadian Journal of Experimental Psychology. 2001;55:164–175. doi: 10.1037/h0087363. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:159–165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Moulin CJA, Perfect TJ, Jones RW. Evidence for intact memory monitoring in Alzheimer’s disease: Metamemory sensitivity at encoding. Neuropsychologia. 2000a;38:1242–1250. doi: 10.1016/s0028-3932(00)00037-3. [DOI] [PubMed] [Google Scholar]
- Moulin CJA, Perfect TJ, Jones RW. Global predictions of memory in Alzheimer’s disease: Evidence for preserved metamemory monitoring. Aging, Neuropsychology, and Cognition. 2000b;7:230–244. [Google Scholar]
- Nelson TO. Consciousness and metacognition. American Psychologist. 1996;51:102–116. [Google Scholar]
- Nelson TO, Narens L. Metamemory: A theoretical framework and new findings. In: Bower GH, editor. The psychology of learning and motivation. New York: Academic Press; 1990. pp. 125–173. [Google Scholar]
- Pannu JK, Kaszniak AW. Metamemory experiments in neurological populations: A review. Neuropsychology Review. 2005;15:105–130. doi: 10.1007/s11065-005-7091-6. [DOI] [PubMed] [Google Scholar]
- Pariente J, Cole S, Henson R, Clare L, Kennedy A, Rossor M, et al. Alzheimer’s patients engage an alternative network during a memory task. Annals of Neurology. 2005;58:870–879. doi: 10.1002/ana.20653. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Relaxing decision criteria does not improve recognition memory in amnesic patients. Memory & Cognition. 1999;27:501–511. doi: 10.3758/bf03211544. [DOI] [PubMed] [Google Scholar]
- Rhodes MG, Jacoby LL. On the dynamic nature of response criterion in recognition memory: Effects of base rate, awareness, and feedback. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:305–320. doi: 10.1037/0278-7393.33.2.305. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Verfaellie M, Anes MD, Racine C. When true recognition suppresses false recognition: Evidence from amnesic patients. Journal of Cognitive Neuroscience. 1998;10:668–679. doi: 10.1162/089892998563086. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology, General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Stretch V, Wixted JT. On the difference between strength-based and frequency-based mirror effects in recognition memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:1379–1396. doi: 10.1037//0278-7393.24.6.1379. [DOI] [PubMed] [Google Scholar]
- Souchay C, Isingrini M, Gil R. Alzheimer’s disease and feeling-of-knowing in episodic memory. Neuropsychologia. 2002;40:2386–2396. doi: 10.1016/s0028-3932(02)00075-1. [DOI] [PubMed] [Google Scholar]
- Swick D, Knight RT. Contributions of prefrontal cortex to recognition memory: electrophysiological and behavioral evidence. Neuropsychology. 1999;13:155–170. doi: 10.1037//0894-4105.13.2.155. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Giovanello KS, Keane MM. Recognition memory in amnesia: Effects of relaxing response criteria. Cognitive Affective Behavioral Neuroscience. 2001;1:3–9. doi: 10.3758/cabn.1.1.3. [DOI] [PubMed] [Google Scholar]
- Windmann S, Urbach TP, Kutas M. Cognitive and neural mechanisms of decision biases in recognition memory. Cerebral Cortex. 2002;12:808–817. doi: 10.1093/cercor/12.8.808. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Stretch V. The case against a criterion-shift account of false memory. Psychological Review. 2000;107:368–376. doi: 10.1037/0033-295x.107.2.368. [DOI] [PubMed] [Google Scholar]