Abstract
Exposure to psychological trauma is common and predicts long-term physical and mental health problems, even in those who initially appear resilient. Here, we used multimodal neuroimaging in healthy adults who were at different distances from the World Trade Center on 9/11/01 to examine the neural mechanisms that may underlie this association. More than three years after 9/11/01, adults with closer proximity to the disaster had lower gray matter volume in amygdala, hippocampus, insula, anterior cingulate, and medial prefrontal cortex, with control for age, gender, and total gray matter volume. Further analysis showed a nonlinear (first-order quadratic) association between total number of traumas in lifetime and amygdala gray matter volume and function in the whole group. Post hoc analysis of subgroups with higher versus lower levels of lifetime trauma exposure revealed systematic associations between amygdala gray matter volume, amygdala functional reactivity, and anxiety that suggest a nonlinear trajectory in the neural response to accumulated trauma in healthy adults
Introduction
More than half of the adults in the United States experience one or more psychological traumas in their lifetime (Kessler et al., 1995). Higher levels of trauma exposure occur in certain subgroups, e.g., those who live in unsafe neighborhoods (Boothroyd and Evans, 2001), or who are exposed to terrorism (Galea et al., 2002) or war (Bramsen et al., 2000). Although only a small percentage of these people develop posttraumatic stress disorder (PTSD) (Kessler et al., 1995), trauma exposure predicts lifetime increases in mental health disorder in the general population (Brown, 1993; Kendler et al., 2003; R. Kessler et al., 1995). Even resilient trauma-exposed individuals are likely to show heightened cardiovascular reactivity to trauma reminders (Tucker et al., 2007) and to have greater vulnerability to PTSD with subsequent trauma exposure (Bremner et al., 1993). Resilient individuals may also experience cognitive declines (Stein et al., 2002), altered catecholamine levels (Otte et al., 2005; Young and Breslau, N, 2004), more chronic illness later in life, and decreased mean life expectancy (McFarlane, 1997). However, the neural mechanisms that may underlie the effects of trauma in this population remain unclear.
Research using animal models has provided some evidence of the effects of stressor exposure on neuronal structure and function. This research suggests that the amygdala, hippocampus, and medial prefrontal cortex may be especially vulnerable (McEwen, 2005; Mitra et al., 2005; Vyas et al., 2002). In general, uncontrollable stressors have been shown to produce hyperexcitability in the amygdala and increased fearful behavior (Adamec et al., 2005; Maier and Watkins, 1998; Rosen and Schulkin, 1998). Acute (Mitra et al., 2005) and chronic (Vyas et al., 2002) restraint stress have produced increased anxiety and increased dendritic spine density in the basolateral amygdala. Chronic restraint stress has been shown to produce hypertrophy of the dendritic arborization in the amygdala, accompanied by dendritic atrophy and decreases in spine density in medial prefrontal areas (Radley et al., 2006) and the hippocampus (Vyas et al., 2002). Severe social stress has produced similar dendritic remodeling in the hippocampus (Blanchard et al., 1993; McKittrick et al., 2000). In addition, chronic stress may reduce neurogenesis in the hippocampus (Gould et al., 1998). In general, these stress-induced neuronal changes tend to recover with rest in prefrontal and hippocampal areas (Heine et al., 2004; McEwen, 2005; Vyas et al., 2004) but changes to the amygdala (and the accompanying fearful behaviors) seem to be more persistent (Adamec et al., 2005; Vyas et al., 2004).
The terrorist attacks of 9/11/01 provide a unique window into these processes in humans, because closer proximity to this disaster has been shown to be associated with increased psychological distress (Blanchard et al., 2004; Galea et al., 2002) and increased amygdala activity (Ganzel et al., 2007; Sharot et al., 2007). In the present study, we measured gray matter volume and functional amygdala activity during passive viewing of fearful versus calm faces (Breiter et al., 1996) in healthy adults who were at different distances from the World Trade Center (WTC) on 9/11/01. We also measured lifetime trauma exposure, state anxiety, and current PTSD symptoms. More than three years after 9/11/01, adults with closer proximity to the WTC had lower gray matter volume in multiple brain regions, including the amygdala. Amygdala gray matter volume was inversely correlated with amygdala response to emotional faces. Lifetime trauma exposure was related to amygdala gray matter volume according to a first-order quadratic, with the slope of the relationship reversing at higher levels of trauma exposure. Analysis of subgroups with higher versus lower levels of lifetime trauma exposure revealed systematic associations between amygdala gray matter volume, amygdala functional reactivity, and anxiety that suggest a nonlinear trajectory in the neural response to accumulated stress.
Materials and Methods
Participants
The study included 36 healthy adults who were either within 1.5 miles of the World Trade Center on 9/11/01 (9/11-exposed) or were living more than 200 miles away at the time and who subsequently moved to the New York Metropolitan area (comparison group). Subjects were screened for contraindications for fMRI and to exclude psychiatric, endocrine, neurological, and other major medical illness (including current PTSD or major depression). People who had friends or relatives on aircraft involved in the 9/11 disaster were excluded, as were those who lived in Washington, D.C., on 9/11/01. Data were collected between 41 and 48 months after 9/11/01. Following optimized VBM analysis, two subjects’ data showed mean global gray matter densities > 2 SD from the mean. These subjects were excluded and the VBM analyses were re-run for a sample size of 34; 17 were 9/11-exposed (6 female, ages 29.7 ± 2.0 years [mean ± standard error]) and 17 were included in the comparison group (8 female, ages 29.2 ± 2.3 years). FMRI data from 12 participants were excluded due to technical difficulties with the scanner (n = 8) or rapid head movement > 1mm (n = 4). Subject recruitment continued until an adequate group size for fMRI analyses was obtained, with a comparison group matched for age and sex. The fMRI sample included eleven 9/11-exposed adults (5 female; ages 30.3 ± 2.5 years) and eleven comparison adults (5 female; ages 29.3 ± 1.4 years). There was no difference in age, sex, or group membership (9/11-exposed versus comparison) between excluded subjects and those included in the fMRI data analyses. This investigation was conducted within institutional guidelines established for protection of human subjects. All participants provided informed written consent.
Behavioral assessments
Lifetime incidence of trauma exposure, current PTSD, and PTSD symptoms at worst trauma were assessed using the PTSD module of the University of Michigan Composite International Diagnostic Interview (Kessler et al., 1994) in conjunction with the Life History Calendar methodology (Caspi et al., 1998). The UM-CIDI is a fully structured diagnostic interview that allows the comprehensive (Breslau et al., 1998; Kessler et al., 1995; E. Young, and Breslau, N, 2004; E. Young and Breslau, 2004) assessment of current and lifetime mental disorders in the form of the third revised Diagnostic and Statistical Manual for Mental Disorders. The UM-CIDI uses the epidemiological definition of trauma, which does not require experience of overwhelming shock, horror, or fear at time of trauma. Approximately 30 minutes elapsed between time of interview and time of scan. An assessment of current PTSD symptoms was obtained using the Impact of Events Scale (Horowitz, 1979), a 15-item measure of PTSD stress reactions in the seven days prior to scanning. Assessment of state anxiety was conducted using the Speilberger State Trait Anxiety Inventory (Spielberger, 1973), a 20-item measure of current state anxiety. Group differences were assessed via t-test using SPSS (SPSS, Inc., Chicago, Ill).
Image acquisition
After a three-plane localizer and a whole-head coronal localizer, a T2*-weighted 2-dimensional anatomical image with a fast spin-echo (FSE) sequence was acquired: TR = 4000, TE = 68 ms, flip = 90°, FOV = 20, 29 slices, 5 mm slice thickness, 0 mm gap, matrix = 256 × 192, axial-oblique. A three-dimensional spoiled gradient recalled (SPGR) T1-weighted anatomic scan was also acquired (124 axial slices, TR = 25 ms, TE = 5 ms, flip = 20°, FOV = 24 cm, 1.5mm thickness, 0 mm gap, matrix = 256 × 256 × 160). Functional data was acquired using a spiral in-out sequence (Preston, Thomason, Ochsner, Cooper, & Glover, 2004) and the same spatial prescription as the FSE: TR = 2000 ms, TE = 30 ms, matrix = 64 × 64 mm, 29 slices per volume.
Voxel-based morphometry (VBM) processing and analysis
Structural images were preprocessed for VBM analysis using “optimized” VBM protocol (Good et al., 2001) with the VBM toolbox in SPM 2 (Wellcome Department of Neurology, London, UK) implemented in MatLab 7.0. Study-specific T1 gray matter, white matter and CSF templates were created from the 3D SPGR T1 scan from all 34 subjects. These template images were then used for segmentation and normalizations of the original 3D SPGR images for each participant using Montréal Neurological Institute (MNI) templates. Template creation and subsequent segmentation and normalization were performed using default options in VBM toolbox (25 mm cut off, medium regularization, medium HMRF [Hidden Markov Random Field]) weighting for segmentation) with 16 nonlinear iterations. The normalized segmented images were modulated and then smoothed with a filter of 12mm FWHM (full width at half maximum) Gaussian kernel. Individual differences in total gray matter volume were controlled in subsequent statistical analysis. Because of this normalization for mean global gray matter differences, the analyses reported here will detect regionally specific differences within the gray matter compartment rather than large-scale variations (Boddaert et al., 2006). For whole brain statistical analyses, clusters of significant VBM difference were reported using a p < 0.001 (uncorrected) threshold and a voxel-extent threshold of more than 150 voxels. Normalized, modulated, smoothed gray matter images were analyzed using ANOVA analysis in SPM 2, comparing the 911-exposed group and the comparison group. Region of interest (ROI) analyses of gray matter volume for amygdala and hippocampus were defined using the Wake Forest University (WFU) PickAtlas (Maldjian et al., 2003). The associations between behavioral measures and the ROI data for gray matter volume were examined using multiple regression analyses performed using SPSS (SPSS, Inc., Chicago, Ill).
fMRI task procedure
Functional magnetic resonance imaging (fMRI) data were obtained in the same session during the passive viewing of fearful versus calm faces within a block paradigm, a standard assay of amygdala responsiveness (Breiter et al., 1996). Participants viewed gray-scaled pictures of faces of eight actors demonstrating fearful and calm facial expressions in a standardized set of pictures of emotional faces. Details of stimuli and presentation are provided elsewhere (Ganzel et al., 2007). Order of presentation was counterbalanced across subjects and across runs using the two following sequences: +FC+FC+FC or +CF+CF+CF (where F indicates a block of fearful faces, C indicates a block of calm faces, and + indicates fixation). In each block of faces, 10 images were presented for 4 s each. Fixation blocks were 30s. Total block duration was 330s.
fMRI data analysis
Functional imaging data was preprocessed and statistical analysis was performed using Statistical Parametric Mapping (SPM2: Wellcome Department of Neurology, London, UK) implemented using MatLab 7.0. fMRI data were normalized to the MNI template and spatially smoothed using a 6mm full-width/half-maximum kernel. Individual level analysis was performed using a fixed effects model (Friston et al., 1995). For each individual, contrast images were constructed comparing each of the block types. These individual-level contrasts served as the basis for group-level random effects analyses (Friston et al., 1995). A one-way ANOVA was conducted to compare brain activation across groups (9/11-exposed and control) for fearful versus calm faces. ROI’s were defined functionally as the voxels found to be reliably activated in the whole-brain analysis of the fearful versus calm contrast at thresholds of p < .01, with cluster extents of three or more contiguous voxels (Ganzel et al., 2007). The association between behavioral measures, gray matter volume, and BOLD signal change in the amygdala for the fearful-calm contrast in each ROI was examined using multiple regression analyses performed using SPSS (SPSS, Inc., Chicago, Ill).
Results
9/11-exposed versus comparison group
We found no significant differences via t-test between the 9/11-exposed and comparison groups in terms of age, sex, age at first trauma, years since most recent trauma, number of traumas in lifetime, number of traumas in lifetime with shock, terror, or horror, or past history of PTSD (Table 1). This was the case with or without the inclusion of two very high trauma subjects in the comparison group (trauma exposure truncated at 30). The 9/11-exposed group experienced their worst trauma more recently than the comparison group: t(30) = 3.77, p = .001 (individuals without trauma exposure were excluded from this analysis). While not suffering from PTSD, this group reported a significantly higher number of current symptoms of PTSD, as measured on the Impact of Events scale (Horowitz, 1979): t(30) = -2.51, p = .02. “Optimized” voxel-based morphometry (VBM) analysis of gray matter volume1 showed regional decreases in gray matter in the 9/11-exposed group in multiple areas (Fig. 1, Table 2), including anterior cingulate cortex, medial prefrontal cortex, bilateral insula, left anterior hippocampus, and left amygdala. We observed no significant regional increases in gray matter volume in the 9/11-exposed group relative to the comparison group.
Table 1.
Comparison of demographic, trauma, and behavioral variables across study groups.
| Control
n = 17 |
9/11 Exposed
n = 17 |
|
|---|---|---|
| Mean (SE) | Mean (SE) | |
| Age at scan (years) | 29.12 (1.2) | 29.6 (2.0) |
| Sex | 8F, 9M | 6F, 11M |
| Age at first trauma | 12.5 (1.4) | 16.9 (1.9) |
| Years since most recent trauma | 5.2 (1.3) | 2.9 (0.4) |
| Years since worst trauma | 12.1 (1.9) | 4.7 (0.9)** |
| Number of traumas in lifetime | 6.2 (2.3) | 4.7 (0.8) |
| Number of traumas in lifetime with shock/ horror | 3.8 (1.1) | 3.6 (0.7) |
| State-Trait Anxiety Inventory (STAI) | 28.8 (1.0) | 29.4 (1.5) |
| Current symptoms of PTSD (Impact of Events Scale) | 16.1 (0.5) | 22.4 (2.2)* |
| Prior history of PTSD | .19 (0.1) | .35 (0.2) |
p < .05;
p <.01. PTSD, posttraumatic stress disorder.
Figure 1.
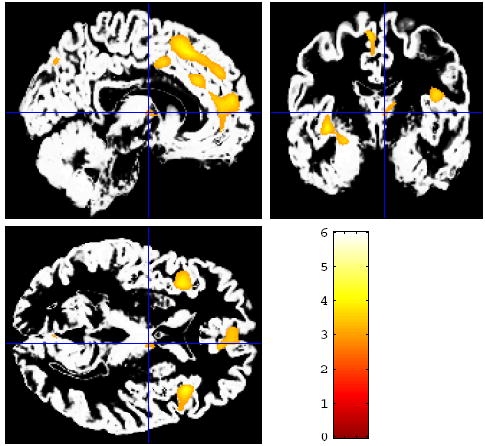
More than three years after the terrorist attacks, whole-brain voxel-based morphometry (VBM) shows multiple areas with significantly lower mean gray matter volume in healthy adults who were within a mile and a half of the World Trade Center on 9/11/01 relative to the comparison group, controlling for age, sex, and total gray matter volume. Regions with lower gray matter volume in the 9/11-exposed group included anterior cingulate, medial prefrontal cortex, insula, amygdala, and anterior hippocampus (Table 2).
Table 2.
| Region | Maximally activated voxel
coordinates |
Z score | Contiguous
Voxels |
|||
|---|---|---|---|---|---|---|
|
Control > 9/11 (all p. >001 uncorrected)
| ||||||
| L Precuneus | -7 | -80 | 39 | 4.84 | 6991 | |
| R Cingulate Gyrus | 8 | 4 | 43 | 3.77 | 198 | |
| R Medial Frontal Gyrus | 7 | 23 | 49 | 3.70 | 1075 | |
| R Inferior Frontal Gyrus | 39 | 21 | 5 | 3.67 | 335 | |
| R Cuneus | 17 | -91 | 33 | 3.66 | 263 | |
| R Superior Parietal Lobule | 22 | -66 | 56 | 3.65 | 660 | |
| L Insula | -37 | -5 | -5 | 3.61 | 306 | |
| L Insula | -39 | 18 | 4 | 3.59 | 729 | |
| R Medial Frontal Gyrus | 5 | 53 | 13 | 3.54 | 996 | |
| R Insula | 43 | -14 | 20 | 3.46 | 453 | |
| L Amygdala, Anterior Hippocampus | -25 | -9 | -14 | 3.55 | 196 | |
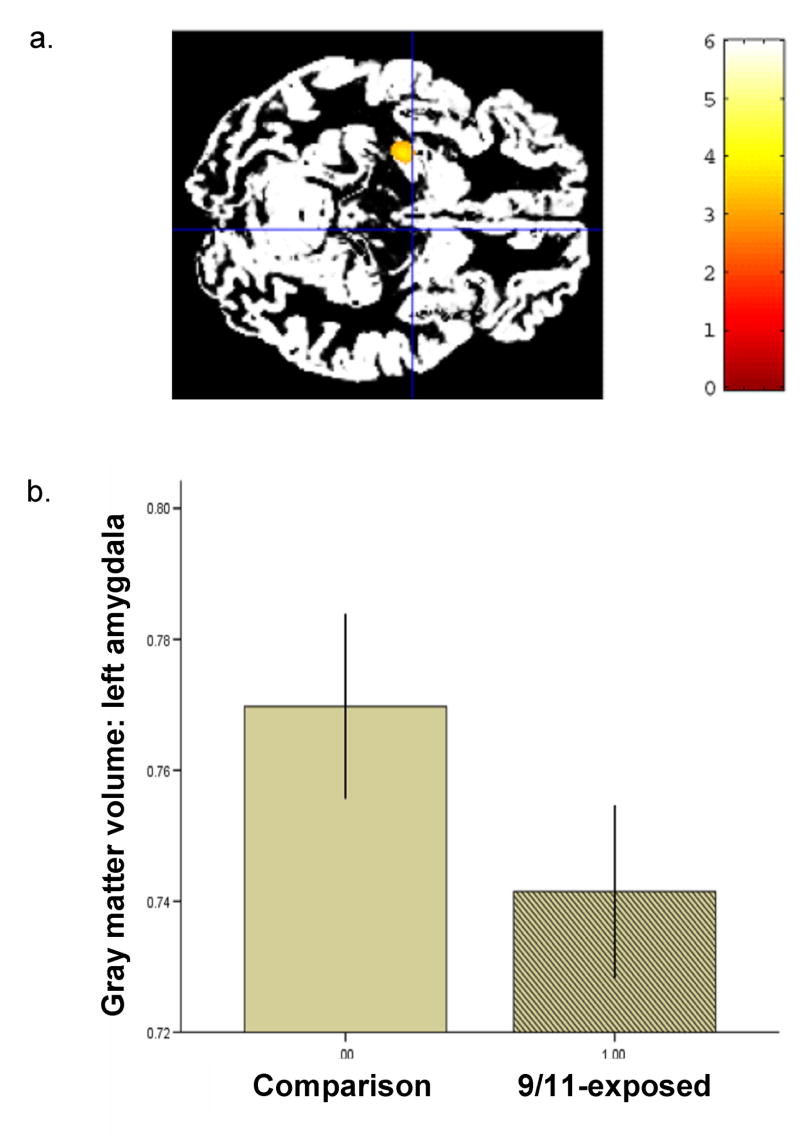
Region of interest (ROI) analyses of left amygdala using standardized neuroanatomical masks (Maldjian et al., 2003) confirmed lower gray matter volume in this region in the 9/11-exposed group relative to the comparison group: β = -.50, p = .005, with control for age and sex (Fig. 2). Time since worst trauma did not contribute significantly to this analysis, nor was there an independent relationship between amygdala gray matter volume and time since worst trauma.
Figure 2.
Amygdala gray matter volumes in the 9/11-exposed group relative to the comparison group. (a) Voxel-wise analysis of variance showed significantly decreased amygdala gray matter volume in the 911-exposed group relative to the comparison group. (MNI -25, -11, -14; Z = 3.55). (b) Region of interest analysis, controlling for age and sex, showed lower mean gray matter volume in the left amygdala in 9/11-exposed group than in the comparison group. Error bars, s.e.m.
Analysis of both groups together showed that gray matter volume in the left amygdala was significantly and inversely correlated with blood oxygen level-dependent (BOLD) signal change in the left amygdala (MNI -25, -9, -20) in response to fearful versus calm faces: β = -.53, p = .02, with control for age and sex (Fig. 3). The significance of this relationship is unaffected by control for number of traumas, years since most recent trauma, and years since worst trauma. This correlation is significant in the 9/11 group (r = -.70, p = .02) and not in the comparison group (r = .38, p > .10).
Figure 3.
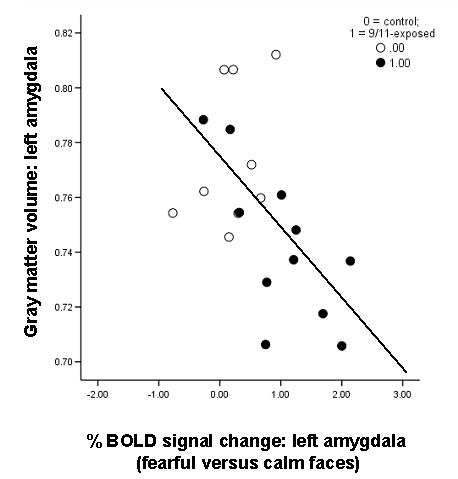
Multimodal imaging data for amygdala showing a negative correlation between amygdala gray matter volume and amygdala BOLD signal change to fearful versus calm faces in the whole group (β = -.53, p = .02, with control for age and sex). Open circles indicate comparison group; closed circles indicate 9/11-exposed.
Accumulated lifetime trauma
In both groups together, regression analysis identified a significant nonlinear relationship between amygdala gray matter volume and number of traumas in lifetime. This relationship took the form of a first-order quadratic, with a negative slope at lower levels of lifetime trauma exposure that reversed at higher levels (Fig. 4): β(number of traumas2) = 1.3, p = .03. Thus, gray matter volume was lowest for those with moderately high levels of trauma exposure (i.e., near the quadratic inflection point at 5 lifetime traumas). This quadratic relationship held in the comparison group alone (β[number of traumas2] = 2.45, p = .02), indicating that this effect may be generalizable to a variety of types of trauma. Control for age, sex, prior history of PTSD, or time since worst trauma did not affect the significance of this association.
Figure 4.
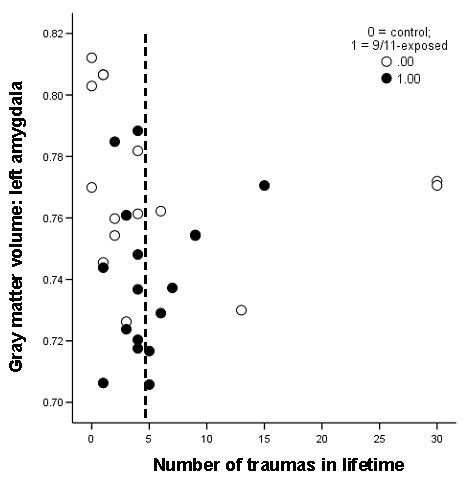
The relationship between amygdala gray matter volume and number of traumas in lifetime. This relationship took the form of a first-order quadratic, such that for lower numbers of lifetime traumas amygdala gray matter volume decreased with increased trauma exposure and for high numbers (greater than 5) the relationship reversed. This relationship was evident in the entire group (β(number of traumas2) = 1.3, p = .03) as well as in the comparison group alone (β[number of traumas2] = 2.45, p = .02). The dotted line indicates the inflection point of the quadratic at 5 traumas in a lifetime. Open circles indicate comparison group; closed circles indicate 9/11-exposed.
To examine this relationship further, the data set was split at the inflection point of the quadratic relationship between amygdala gray matter volume and number of traumas in lifetime (n = 24 for 0-5 traumas in lifetime; n = 10 for more than 5 traumas in lifetime). Post hoc analysis of the functional imaging and behavioral data in these higher and lower trauma exposure subgroups revealed that the multimodal imaging data and behavioral data were inversely related within each subgroup (Suppl. Figs. 1 & 2). In the group with five or fewer traumas, linear regression identified negative slopes in the relationships between amygdala gray matter volume and both state anxiety and current symptoms of PTSD. In the group with more than five traumas in lifetime, the slopes of these relationships were positive (Suppl. Fig. 1). A similar, but opposite, pattern was seen within the fMRI data for the amygdala. Among those with five or fewer traumas, there were positive slopes in the relationships between BOLD signal change in the left amygdala to fearful versus calm faces and measures of anxiety. The slopes of the relationships between these factors again reversed for those with more than 5 traumas (Suppl. Fig. 2). Level of lifetime trauma exposure was found to interact with amygdala gray matter volume and amygdala function to predict anxiety in the group as a whole, which provided a measure of the significance of this change in regression slopes at different levels of trauma exposure. Specifically, higher versus lower levels of lifetime trauma exposure significantly interacted with amygdala gray matter volume (β = 21.40, p = .004, with Bonferroni correction for multiple comparisons) and amygdala BOLD signal reactivity to emotional faces (β = -.73, p = .006, corrected) in separate linear regression models with state anxiety as the dependent variable. Interactions were not significant between levels of lifetime trauma exposure and amygdala gray matter volume and BOLD signal reactivity in the prediction of symptoms of PTSD, with correction for multiple comparisons. This may follow from low reporting of symptoms at higher levels of trauma exposure. Overall, this pattern of relationships suggests that there may be qualitative differences in the associations between amygdala volume and functional response to emotional stimuli and behavioral indicators of anxiety at lower versus higher levels of lifetime trauma exposure.
Hippocampus
The area of gray matter decrease that involved much of the left amygdala also extended to include a portion of the left anterior hippocampus. ROI analyses using standardized neuroanatomical masks (Maldjian et al., 2003) of the left hippocampus revealed a small decrease in mean hippocampal volume in the 9/11-exposed group relative to the comparison group: t(33) = 2.19; p = .03. Linear regression analyses showed that gray matter volume in the hippocampus was correlated with gray matter volume in the amygdala, with control for age and sex: β = .57, p < .000. Further analyses indicated that the relationships between hippocampal volume and number of traumas had a similar form to those seen with the amygdala, but they were not significant. It may be that part of the anterior hippocampus has a similar relationship with trauma as the amygdala; however, the ROI used here covered the entire left hippocampus, which may have diluted variation in anterior hippocampus below the threshold for significance in these analyses.
Age
Increasing age was correlated with number of traumas in lifetime in this sample: r = .33, p = .04. Age showed a nonlinear relationship with amygdala gray matter volume: β(age2) = -3.96, p = .02. Gray matter volume increased with age to approximately age 30 years and began to decrease afterwards. There was also a trend of a similar inverse relationship (minimum at approximately age 30) with amygdala BOLD signal reactivity to emotional faces: β(age2) = 4.09, p = .06. However, control for age (both linear and quadratic terms) did not change the strength of the relationship between number of traumas in lifetime and amygdala gray matter volume nor any of the interactions in the models assessed above. This suggests that number of traumas in lifetime and age have independent effects on amygdala structure and functional response in this sample.
Sex
The above analyses were recomputed including sex. Sex did not make a significant independent contribution to any of these models, nor did its inclusion make a significant difference in the strength of any of the above interactions. This suggests that sex was not a significant factor in these analyses; however the relatively small sample size precludes a definitive conclusion on this point.
Discussion
More than three years after 9/11/01, adults who had greater proximity to the terrorist attacks on the World Trade Center had significantly lower gray matter volume in amygdala, anterior hippocampus, insula, anterior cingulate, and medial prefrontal cortex. The 9/11-exposed group had no areas of significantly increased gray matter relative to the comparison group. All study subjects were free of mental and physical health disorder, so the regional differences in gray matter volumes observed here are not attributable to the presence of clinical disorder. These results provide evidence of structural change in gray matter in healthy adults following psychological trauma.
Examination of the amygdala as a region of interest confirmed the decrease in amygdala gray matter volume in the 9/11-exposed group. In general, these analyses also showed that decreases in amygdala gray matter volume were associated with increased BOLD signal change in the left amygdala in response to fearful versus calm faces. Leftward lateralization is consistent with reports that the left amygdala is more likely to be activated in response to tasks involving the neural processing of negative emotional stimuli (Baas, Aleman, & Kahn, 2004; Wager, Phan, Liberzon Taylor, 2003) that involve detailed evaluation of emotional intensity (Glascher & Adolphs, 2003). Further analysis of the relationship between amygdala function and structure and lifetime trauma exposure revealed significant differences between those with fewer traumas (≤ 5) throughout their lifetime and those with many traumas (> 5). Overall, the relationship between lifetime trauma exposure and amygdala gray matter volume took the form of a first-order quadratic, such that the slope of this relationship reversed at higher levels of trauma exposure. This held for the comparison group alone, as well as for the sample as a whole.
Analyses of lower and higher lifetime trauma subgroups helped to clarify associations between behavioral measures of anxiety and both the structural and functional imaging data for the amygdala. In the group with five or fewer traumas in their lifetime (70% of the sample), state anxiety increased as amygdala gray matter volume decreased. Among those with more than five traumas, this relationship was reversed; anxiety increased as amygdala gray matter volume increased. This produced a significant interaction between level of lifetime trauma exposure and amygdala gray matter volume in predicting state anxiety. Consistent with the inverse relationship between gray matter volume and amygdala functional reactivity in this sample, we found an interaction between level of trauma exposure and amgydala BOLD signal (fearful versus calm faces) in predicting anxiety that was the inverse of the previous interaction. Among those with five or fewer traumas, anxiety increased with increasing BOLD signal to fearful versus calm faces – however, it decreased with increasing BOLD signal in those with five or more traumas. The relationships between symptoms of PTSD, level of trauma exposure, and amgydala gray matter and response to emotional faces took a similar, although nonsignificant, form. These results require replication with a larger sample of individuals who have more than five traumas in their lifetime. However, they do suggest that there may be qualitative differences among those with higher versus lower levels of trauma exposure in the relationships between behavioral anxiety and amygdala structure and reactivity. These data also provide supporting evidence for a nonlinear trajectory in amygdala stress-related neural plasticity with accumulating trauma exposure.
The use of VBM allowed us to perform a bias-free analysis of gray matter differences across the whole brain. Because VBM allows comparison of the local composition of tissue, it is useful for showing group differences in structure that may be more subtle than traditional volumetric measurement can resolve. VBM analyses are limited by an inability to interpret the nature of the specific microstructural changes that may be measured (e.g., changes in neuropil, neuronal size, dendritic, or axonal arborisation) (Ashburner and Friston, 2001). This issue remains unresolved and will require future studies that utilize methods other than MRI. Thus, we are unable to know what specific types of gray matter changes were associated with trauma exposure in this study. VBM analysis also includes possible confounds related to the normalization and segmentation process, which can be especially problematic in comparisons of atypical populations (Bookstein, 2001). To help address these concerns, we used an “optimized” VBM procedure, set significance levels arbitrarily high, and included only healthy subjects for whom no group differences in global brain shape would be expected. Because VBM analyses may be less sensitive to volume loss in the amygdala than traditional region-of-interest volumetric measurement (Good et al., 2002), the results reported here may be conservative.
These findings suggest multiple avenues for future research. Most of the brain regions identified here as having lower gray matter volume in the 9/11-exposed group (amygdala, insula, anterior cingulate, and medial prefrontal cortex) have been identified as playing key roles in the evaluation and regulation of emotional stimuli in humans (Ochsner et al., 2004; Phan et al., 2002), suggesting that traumatic experiences may specifically affect the neural processing and regulation of emotion in nonclinical populations. This possibility clearly requires further study. Assessment of participants’ prior trauma exposure may be an important inclusion in studies of the neural correlates of emotion processing and regulation, and exploration of possible effects of stress-related neural change on emotion regulation may also help shed light on the emerging neuroscience of emotion-cognition interactions (Ochsner and Phelps, 2007).
In addition, we report trauma-related decreases in gray matter in many of the same brain regions in which gerontologists report normal age-related gray matter atrophy (Good et al., 2001; Resnick et al., 2003). The established prevalence of trauma exposure in the general population (Breslau et al., 1998; Kessler et al., 1995) suggests the possibility that some of the gray matter atrophy attributed to aging may instead be due to accumulated lifetime trauma exposure. It would be of interest to delineate the relative contribution of age versus lifetime trauma exposure to the gray matter atrophy seen in older adults. Studies of aging samples have also identified increased BOLD signal change to emotional faces (Wright et al., in press) and decreased amygdala volume (Shiino et al., 2006) in those with mild Alzheimer’s disease. In the present study, we found decreased amygdala gray matter volume and increased amygdala functional reactivity to emotional stimuli in subjects with moderately high levels of lifetime trauma exposure (approximately 4 to 6 traumas). It would be of interest to examine whether stress-related changes in the amygdala represent a vulnerability factor for Alzheimer’s disease or conversely, if these differences may be attributable entirely or in part to increased stress that is associated with disease onset.
Amygdala gray matter atrophy, often accompanied by hippocampal volume atrophy, has also been reported in depression (Campbell et al., 2004; Siegle, Konecky, Thase, Carter, 2003; Sheline et al., 1999), borderline personality disorder (Schmahl et al., 2003), and anxiety disorder (Milham et al., 2005). There are also reports of increased amygdala reactivity to emotional stimuli in these populations (Siegle et al., 2003; Schmahl et al., 2003; Sheline et al., 2001; Thomas et al., 2001). For example, amygdala hyperactivity in depression (Drevets et al., 1992; Sheline et al., 2001) appears to be associated initially with amygdala hypertrophy (Frodl et al., 2002), followed by amygdala atrophy after multiple episodes of major depression (Sheline et al., 1999). Drawing on animal models of chronic stressor exposure, it has been argued (McEwen, 2003) that chronic hyperexcitability of the amygdala may produce these progressive alterations in amygdala volume over time, possibly through the influence of excitatory amino acids on cell survival and neuronal architecture.” In the present study, we find that decreases in amygdala and hippocampal gray matter coupled with increased amygdala reactivity are associated with moderately high levels of lifetime trauma exposure. Because of the relatively high incidence of trauma exposure in the general population (Kessler et al., 1995), it may be that trauma exposure plays a common explanatory role in some portion of the observed neural differences in the medial temporal lobe in these disorders. Consideration of nonlinearity in these relationships may help to clarify some of the inconsistencies in this literature (Campbell et al., 2004).
A negative correlation between amygdala gray matter volume and BOLD signal in the amygdala for fearful versus calm faces was identified in the 9/11-exposed group but not in the comparison group. This lack of significance is reported with caution because technical difficulties with the scanner (see Methods) caused BOLD data to be lost for a number of subjects who had good quality data for gray matter volume. This narrowed the range of values for amygdala gray matter volume in the comparison group (note the relative ranges of values for gray matter volume in Figures 3 and 4). This loss of data affects only the direct comparison of gray matter volume and BOLD data illustrated in Figure 3. All other analyses reported here employ the full range of gray matter volume data from this sample; within this expanded data set, the effect of anxiety, symptoms, and traumas plotted against amygdala gray matter volume is consistently the inverse of the effect of anxiety, symptoms, and traumas plotted against amygdala BOLD signal (F>C), suggesting an inverse relationship between these two sets of imaging data. Full articulation of this relationship requires replication within a larger sample.
The current analyses control for sex, which may mask real variation in the data. Examination of the relationships between gray matter volume, BOLD signal, and behavioral data within subgroups of males and females was not possible due to the small size of the sample, although we found no significant statistical contribution of sex to the results reported here.
Our findings suggest that trauma exposure plays a causal role in changes to both brain structure and function, even in nonclinical adult populations. These relationships may differ depending on the amount of lifetime trauma exposure a person has experienced. Greater understanding of possible stress-related neural change has the potential to inform a number of disciplines, including the understanding of the alteration in brain function and structure associated with normal aging and a range of mental health disorders, as well current understanding of the socioemotional consequences of stress and trauma exposure. Research is needed to examine whether there is evidence for a similar pattern of neural change with the accumulation of other types of stressor exposure (e.g., poverty, divorce, job loss) and whether these effects are the same or different in children and the elderly. This may, in turn, aid in designing interventions to help keep environmental risk from manifesting as decreased health and well-being across the lifespan.
Supplementary Material
The slope of the relationship between amygdala gray matter volume and state anxiety was negative at low levels of lifetime trauma exposure (a) and reversed at high levels (b). Current PTSD symptoms (c & d) showed a similar change in slopes at higher versus lower levels of trauma exposure. This resulted in an interaction between higher versus lower levels of lifetime trauma exposure and amygdala gray matter volume in predicting state anxiety (β = 21.40, p = .004, corrected). The interaction predicting current symptoms of PTSD was not significant. Open circles indicate comparison group; closed circles indicate 9/11-exposed.
The slope of the relationship between amygdala BOLD response to fearful versus calm faces and state anxiety was negative at low levels of lifetime trauma exposure (a) and reversed at high levels (b). This resulted in an interaction between higher versus lower levels of lifetime trauma exposure and amygdala gray matter volume in predicting state anxiety (β = -.73, p = .006, corrected). Current PTSD symptoms (c & d) showed a similar qualitative shift at higher versus lower levels of trauma exposure. The interaction predicting current symptoms of PTSD was not significant, due to very low reporting of symptoms at higher levels of trauma exposure. Open circles indicate comparison group; closed circles indicate 9/11-exposed.
Acknowledgments
We thank B. J. Casey for guidance during this study. Thanks also to C. Eccard, E. Aronstam, G. Carrigan, M. Henderson, E. Horowitz, J. Katz, A. Levitan, C. Neuendorf, and M. Snow for valuable assistance with this project, and to M.K. Belmonte, B. Finlay, and N. Tottenham for comments on the manuscript. This work was supported by NIH Kirchstein NRSA MH68139 to BG.
Footnotes
Gray matter values for Jacobian modulated voxel-based morphometry (VBM) images are referred to as gray matter volume, a term that reflects the influence of regional volumes on the relative magnitude of the gray matter values, as well as cortical volume averaging (Eckert et al., 2006).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec RE, Blundell J, Burton P. Neural circuit changes mediating lasting brain and behavioral response to predator stress. Neurosci Biobehav Rev. 2005;29:1225–1241. doi: 10.1016/j.neubiorev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. NeuroImage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai BS, McEwen BS, Weiss SM, Blanchard RJ. Subordinate stress: Behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58:113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Margarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review. Brain Res Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Kuhn E, Rowell D, Hickling EJ, Wittrock D, Rogers R, et al. Studies of the vicarious traumatization of college students by the September 11th attacks: Effects of proximity, exposure and connectedness. Behav Res Ther. 2004;42:191–295. doi: 10.1016/S0005-7967(03)00118-9. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Mochel F, Meresse I, Seidenwurm D, Cachia A, Brunelle F, Lyonnet S, Zilbovicius Parieto M. Occipital grey matter abnormalities in children with Williams syndrome. NeuroImage. 2006;30:721–725. doi: 10.1016/j.neuroimage.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. NeuroImage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Boothroyd RA, Evans ME. Environmental safety and exposure to violence of inner city children experiencing a psychiatric crisis. Int J Anthropol. 2001;16:197–209. [Google Scholar]
- Bramsen I, Dirkzwager AJE, van der Ploeg HM. Predeployment personality traits and exposure to trauma as predictors of posttraumatic stress symptoms: A prospective study of form peacekeepers. Am J Psychiatry. 2000;157:1115–1119. doi: 10.1176/appi.ajp.157.7.1115. [DOI] [PubMed] [Google Scholar]
- Breiter H, Etcoff N, Whalen P, Kennedy W, Rauch S, Buckner R, et al. Response and habituation of the human amygdala during visual processing of facial expressions. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick S, Johnson D, Yehuda R, Charney D. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. Am J Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community - The 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998:57. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Brown G. Life events and affective disorder: Replications and limitations. Psychosom Med. 1993;55:248–259. doi: 10.1097/00006842-199305000-00003. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington HL, et al. The Life History Calendar: A research and clinical assessment method for collecting retrospective event-history data. Int J Meth Psychiatry Res. 1998;6:101–114. [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Tenforde A, Galaburda AM, Bellugi U. To modulate or not to modulate: Differing results in uniquely shaped Williams syndrome brains. NeuroImage. 2006;32:1001–1007. doi: 10.1016/j.neuroimage.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical Parametric Maps in Functional Imaging: A General Linear Approach. Human Brain Mapp. 1995;2:189–210. [Google Scholar]
- Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, et al. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Galea S, Ahern J, Resnick H, Kilpatrick D, Bucuvalas M, Gold J, et al. Psychological sequelae of the September 11 terrorist attacks in New York City. New Eng J Med. 2002;346:982–987. doi: 10.1056/NEJMsa013404. [DOI] [PubMed] [Google Scholar]
- Ganzel B, Casey BJ, Voss HU, Glover G, Temple E. The aftermath of 9/11: Effect of recency and intensity on outcome. Emotion. 2007;7:227–238. doi: 10.1037/1528-3542.7.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci. 2003;23:10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell percursors in the dentate gyrus of adult monkeys is diminshed by stress. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Euro J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: A measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiatry. 2003;60(8):789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kessler R, Sonnega A, Bromet E, Hughes M, Nelson C. Posttraumatic stress disorder in the National Comorbidity Study. Arch Gen Psychiatry. 1995;52:1048–1059. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle K, Zhao S, Nelson CB, Hughes M, Eshelman S, et al. Lifetime and 12-month prevalence of DSM-lllR psychiatric disorders in the United States: Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Maier S, Watkins L. Stressor controllability, anxiety, and serotonin. Cog Ther Res. 1998;22:595–613. [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectronic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biological Psychiatry. 2003;54:200–2007. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McFarlane A. The prevalence and longitudinal course of PTSD: Implications for neurobiological models of PTSD. In: Yehuda R, McFarlane A, editors. Psychobiology of Posttraumatic Stress Disorder. Vol. 821. New York Academy of Sciences; NY: 1997. pp. 10–24. [DOI] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, Dickstein DS, Leibenluft E, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: A voxel-based morphometry investigation. Biol Psychiatry. 2005;57:961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Chattarji S. Stress duration modulates the spatioteporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Phelps EA. Emerging perspectives on emotion-cognition interactions. Trends Cogn Sci. 2007;11:317–318. doi: 10.1016/j.tics.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, et al. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biol Psychiatry. 2005;57:27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH. Comparison of Spiral-In/Out and Spiral-out BOLD fMRI at 1.5T and 3T. Neuroimage. 2004;21:291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–329. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, Bremner J. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res Neuroimaging. 2003;122(3):193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals. Ann N Y Acad Sci. 2003;985:481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- Sharot T, Martorella EA, Delgado MR, Phelps EA. How personal experience modulates the neural circuitry of memories of September 11. Proc Natl Acad Sci USA. 2007;104:389–394. doi: 10.1073/pnas.0609230103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatmetn: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shiino A, Watanabe T, Maeda K, Kotani E, Akiguchi I, Matsuda M. Four subgroups fo Alzheimer’s disease based on patterns of atrophy using VBM and a unique pattern for early onset disease. NeuroImage. 2006;33:17–26. doi: 10.1016/j.neuroimage.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the state-trait anxiety inventory for children. Palo Alto, CA: Consulting Psychologists Press; 1973. [Google Scholar]
- Stein MB, Kennedy CM, Twamley EW. Neuropsychological function in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:1079–1088. doi: 10.1016/s0006-3223(02)01414-2. [DOI] [PubMed] [Google Scholar]
- Thomas K, Drevets W, Dahl R, Ryan N, Birmaher B, Eccard C, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Tucker PM, Pfefferbaum B, North CS, Kent A, Burgin CE, Parker DE, et al. Physiologic reactivity despite emotional resilience several years after direct exposure to terrorism. Am J Psychiatry. 2007;164:230–235. doi: 10.1176/ajp.2007.164.2.230. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdalaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D. A functional magnetic resonance imaging study of amygdala response to human faces in aging and mild Alzheimer’s disease. Biol Psychiatry. doi: 10.1016/j.biopsych.2006.11.013. in press. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Young E, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: an epidemiologic community study. Arch Gen Psychiatry. 2004;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Young E, Breslau N. Saliva cortisol in posttraumatic stress disorder: A community epidemiologic study. Biol Psychiatry. 2004;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The slope of the relationship between amygdala gray matter volume and state anxiety was negative at low levels of lifetime trauma exposure (a) and reversed at high levels (b). Current PTSD symptoms (c & d) showed a similar change in slopes at higher versus lower levels of trauma exposure. This resulted in an interaction between higher versus lower levels of lifetime trauma exposure and amygdala gray matter volume in predicting state anxiety (β = 21.40, p = .004, corrected). The interaction predicting current symptoms of PTSD was not significant. Open circles indicate comparison group; closed circles indicate 9/11-exposed.
The slope of the relationship between amygdala BOLD response to fearful versus calm faces and state anxiety was negative at low levels of lifetime trauma exposure (a) and reversed at high levels (b). This resulted in an interaction between higher versus lower levels of lifetime trauma exposure and amygdala gray matter volume in predicting state anxiety (β = -.73, p = .006, corrected). Current PTSD symptoms (c & d) showed a similar qualitative shift at higher versus lower levels of trauma exposure. The interaction predicting current symptoms of PTSD was not significant, due to very low reporting of symptoms at higher levels of trauma exposure. Open circles indicate comparison group; closed circles indicate 9/11-exposed.