Abstract
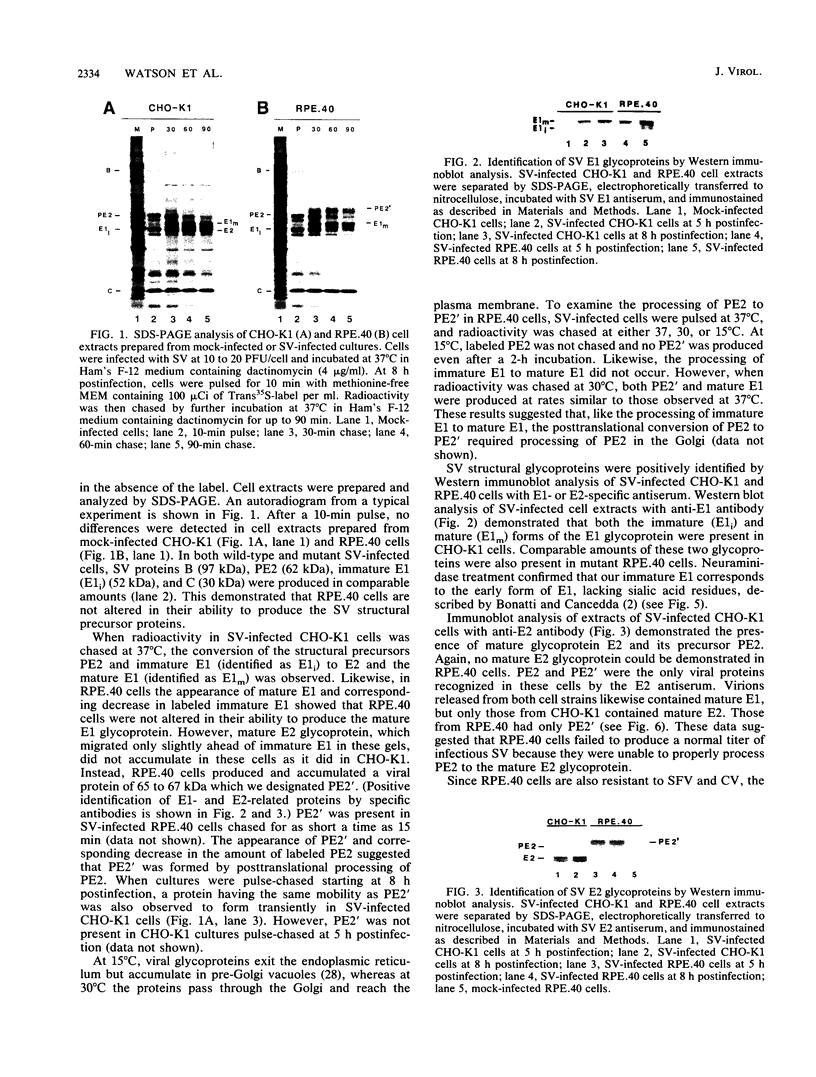
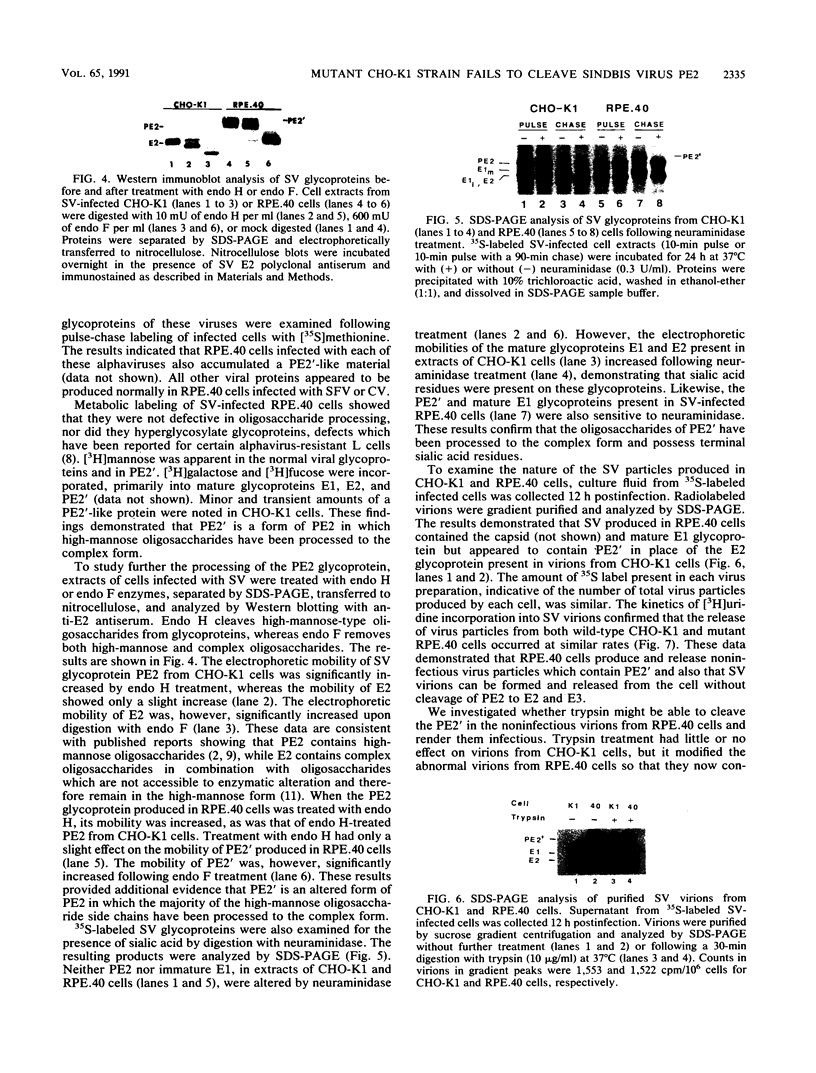
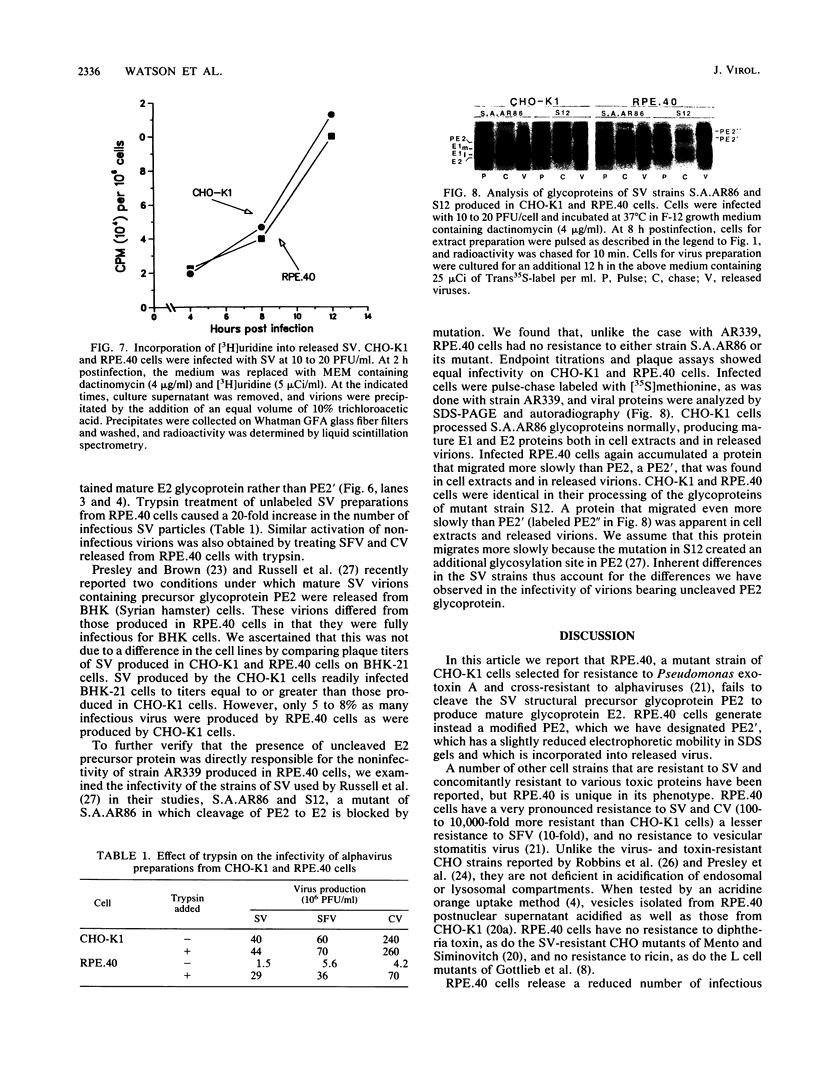
RPE.40, a mutant CHO-K1 strain selected for resistance to Pseudomonas exotoxin A, is defective in the production of infectious alphaviruses, although viruses are taken in and processed normally (J. M. Moehring and T. J. Moehring, Infect. Immun. 41:998-1009, 1983). To determine the cause of this defect, the synthesis of Sindbis virus proteins was examined. RPE.40 cells produced and glycosylated structural glycoprotein precursors PE2 and immature E1 normally. Mature E1 was formed, but PE2 was not cleaved to E2 and E3. PE2 instead was modified to a higher-molecular-weight form (PE2') in which the high-mannose oligosaccharides were processed to the complex form without proteolytic cleavage. The data suggest that the cleavage which produces E2 occurs within the trans-Golgi or in post-Golgi elements and is closely associated with the addition of sialic acid residues to the asparagine-linked oligosaccharides. RPE.40 cells make and release noninfectious Sindbis virions that contain PE2' and no detectable E2. These virions can be converted to an infectious form by treatment with trypsin. A defect in an intracellular endopeptidase activity in RPE.40 cells is postulated. Comparison of two Sindbis virus strains showed that the requirement for E2 in the virion to ensure infectivity is strain specific.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. H., Brown D. T. Inhibition of Sindbis virus maturation after treatment of infected cells with trypsin. J Virol. 1982 Feb;41(2):692–702. doi: 10.1128/jvi.41.2.692-702.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonatti S., Cancedda F. D. Posttranslational modifications of Sindbis virus glycoproteins: electrophoretic analysis of pulse-chase-labeled infected cells. J Virol. 1982 Apr;42(1):64–70. doi: 10.1128/jvi.42.1.64-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Colbaugh P. A., Kao C. Y., Shia S. P., Stookey M., Draper R. K. Three new complementation groups of temperature-sensitive Chinese hamster ovary cell mutants defective in the endocytic pathway. Somat Cell Mol Genet. 1988 Sep;14(5):499–507. doi: 10.1007/BF01534715. [DOI] [PubMed] [Google Scholar]
- Dalgarno L., Rice C. M., Strauss J. H. Ross River virus 26 s RNA: complete nucleotide sequence and deduced sequence of the encoded structural proteins. Virology. 1983 Aug;129(1):170–187. doi: 10.1016/0042-6822(83)90404-x. [DOI] [PubMed] [Google Scholar]
- Davidson S. K., Hunt L. A. Sindbis virus glycoproteins are abnormally glycosylated in Chinese hamster ovary cells deprived of glucose. J Gen Virol. 1985 Jul;66(Pt 7):1457–1468. doi: 10.1099/0022-1317-66-7-1457. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb C., Kornfeld S., Schlesinger S. Restricted replication of two alphaviruses in ricin-resistant mouse L cells with altered glycosyltransferase activities. J Virol. 1979 Jan;29(1):344–351. doi: 10.1128/jvi.29.1.344-351.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi J., Atkinson P. H. Glycosylation of intracellular Sindbis virus glycoproteins. Biochemistry. 1982 Apr 27;21(9):2140–2145. doi: 10.1021/bi00538a024. [DOI] [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Host-dependent variation of asparagine-linked oligosaccharides at individual glycosylation sites of Sindbis virus glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2548–2554. [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Selective cleavage by endo-beta-N-acetylglucosaminidase H at individual glycosylation sites of Sindbis virion envelope glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2555–2561. [PubMed] [Google Scholar]
- Hunt L. A. Sindbis virus glycoproteins acquire unusual neutral oligosaccharides in both normal and lectin-resistant Chinese Hamster ovary cell lines. Virology. 1981 Sep;113(2):534–543. doi: 10.1016/0042-6822(81)90181-1. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney R. M., Johnson B. J., Brown V. L., Trent D. W. Nucleotide sequence of the 26 S mRNA of the virulent Trinidad donkey strain of Venezuelan equine encephalitis virus and deduced sequence of the encoded structural proteins. Virology. 1986 Jul 30;152(2):400–413. doi: 10.1016/0042-6822(86)90142-x. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Knipfer M. E., Brown D. T. Intracellular transport and processing of Sindbis virus glycoproteins. Virology. 1989 May;170(1):117–122. doi: 10.1016/0042-6822(89)90358-9. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mayne J. T., Bell J. R., Strauss E. G., Strauss J. H. Pattern of glycosylation of Sindbis virus envelope proteins synthesized in hamster and chicken cells. Virology. 1985 Apr 15;142(1):121–133. doi: 10.1016/0042-6822(85)90427-1. [DOI] [PubMed] [Google Scholar]
- Mento S. J., Siminovitch L. Isolation and preliminary characterization of Sindbis virus-resistant Chinese hamster ovary cells. Virology. 1981 Jun;111(2):320–330. doi: 10.1016/0042-6822(81)90336-6. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. Strains of CHO-K1 cells resistant to Pseudomonas exotoxin A and cross-resistant to diphtheria toxin and viruses. Infect Immun. 1983 Sep;41(3):998–1009. doi: 10.1128/iai.41.3.998-1009.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D. Activation of precursors to both glycoporteins of Newcastle disease virus by proteolytic cleavage. Virology. 1977 Mar;77(1):125–134. doi: 10.1016/0042-6822(77)90412-3. [DOI] [PubMed] [Google Scholar]
- Presely J. F., Brown D. T. The proteolytic cleavage of PE2 to envelope glycoprotein E2 is not strictly required for the maturation of Sindbis virus. J Virol. 1989 May;63(5):1975–1980. doi: 10.1128/jvi.63.5.1975-1980.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Oliver C., Bateman J. L., Krag S. S., Galloway C. J., Mellman I. A single mutation in Chinese hamster ovary cells impairs both Golgi and endosomal functions. J Cell Biol. 1984 Oct;99(4 Pt 1):1296–1308. doi: 10.1083/jcb.99.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. L., Dalrymple J. M., Johnston R. E. Sindbis virus mutations which coordinately affect glycoprotein processing, penetration, and virulence in mice. J Virol. 1989 Apr;63(4):1619–1629. doi: 10.1128/jvi.63.4.1619-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984 Sep;38(2):535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Scheefers H., Scheefers-Borchel U., Edwards J., Brown D. T. Distribution of virus structural proteins and protein-protein interactions in plasma membrane of baby hamster kidney cells infected with Sindbis or vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7277–7281. doi: 10.1073/pnas.77.12.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Sefton B. M. Two small virus-specific polypeptides are produced during infection with Sindbis virus. J Virol. 1979 Mar;29(3):1186–1195. doi: 10.1128/jvi.29.3.1186-1195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]