Abstract
Objectives
The impact of depressive symptoms on cognitive decline in older adults remains unclear due to inconsistent findings in the literature. It is also unclear whether effects of depressive symptoms on cognitive decline vary with age. This study investigated the effect of concurrent, baseline, and average depressive symptoms on cognitive functioning and decline, and examined the interactive effect of age and depressive symptoms on cognition.
Design
Prospective observational design with examination of cognitive performance and depressive symptoms at 1–2 year intervals for up to 26 years.
Setting
Baltimore Longitudinal Study of Aging, National Institute on Aging.
Participants
1, 586 dementia-free adults 50 years of age and older.
Measurements
Scores over time on the Center for Epidemiologic Studies Depression Scale and measures of learning and memory, attention and executive functions, verbal and language abilities, visuospatial functioning, and general cognitive status.
Results
Increased depressive symptoms were associated with poor cognitive functioning and cognitive decline in multiple domains. Concurrent, baseline, and average depressive symptoms had differential associations with cognition. Average depressive symptoms, a measure of chronic symptoms, appeared to show the most widespread effects on cognitive abilities. Effects of depressive symptoms on some frontal functions were greater with advancing age.
Conclusion
Depressive symptoms are associated with poor cognitive functioning and cognitive decline, particularly with advancing age. The widespread impact of average depressive symptoms on cognition suggests that clinicians should consider the chronicity of depressive symptoms when evaluating cognitive functioning in older adults.
Keywords: aging, sub-clinical depression, cognition
Depression in late life is associated with cognitive deficits beyond the effects of normal aging. Older depressed adults perform more poorly than elderly controls across various neuropsychological domains, including attention, visuospatial abilities, memory processing, concept formation, information processing speed, and overall cognitive functioning (1–4). There is also an inverse association between late-life depression and cognitive function in the sub-clinical symptom range (5, 6). Moreover, there is cross-sectional evidence that the adverse impact of depression on cognition increases as a function of age (3), although some studies have failed to show this effect (1). However, while these observations provide evidence that age may influence the effects of depression on cognitive functioning, it is unclear whether there is a similar interactive effect of age and depression on cognitive decline.
Several longitudinal studies provide evidence that depressive symptoms measured at baseline predict subsequent cognitive decline in older adults (7–9). This association has been observed for general cognitive status, episodic memory, visuospatial ability, processing speed, and executive functions. There is evidence that baseline depressive symptoms predict decline in general cognitive status in participants with persistent, but not episodic, depressive symptoms (4). In contrast, other longitudinal studies have failed to show a relationship between depressive symptoms and cognitive decline (2, 10). Some studies found significant cross-sectional associations between depressive symptoms and cognitive performance in the absence of significant longitudinal findings (10). Other studies reported longitudinal effects of depressive symptoms on information processing speed, but not on memory or other cognitive functions (2). The variability in these longitudinal findings may be due, in part, to sampling differences among these studies. For example, some studies found an association between depressive symptoms and cognitive decline only in groups of people who already evinced poor cognitive functioning (11) or in individuals with higher education (12).
The current study examined the relationship between depressive symptoms, age, and cognitive function and decline in a sample of older adults from the Baltimore Longitudinal Study of Aging (BLSA). We examined 1) the relationship between concurrent depressive symptoms and cognitive performance, 2) whether depressive symptoms at baseline predict subsequent cognitive decline, and 3) whether average depressive symptoms, a measure of chronic or persistent depressive symptoms, are associated with cognitive decline. Additionally, we were interested in whether the effect of depressive symptoms on cognitive function and decline varied as a function of age. We expected chronic depressive symptoms to have a greater impact on cognition than symptoms that are more transient in nature. We further predicted that depressive symptoms would have a greater impact with advancing age for executive functions, processes that are particularly vulnerable to the effects of age and depression (3).
Methods
Participants
Data for the present study were obtained from the Baltimore Longitudinal Study of Aging (BLSA) at the National Institute on Aging, a longitudinal study initiated in 1958. The BLSA sample is a community-dwelling, generally healthy group of participants who volunteer for medical, psychological, and cognitive testing approximately every two years (13). In 2000, individuals 80 and older began returning for testing every year. At enrollment, exclusionary criteria included a history of central nervous system disease (dementia, stroke, Parkinson’s disease, epilepsy, and other neurological conditions), severe cardiac disease (including myocardial infarction, coronary bypass surgery, or angioplasty), and metastatic cancer.
The current study initially included 2,108 volunteers aged 50 years or older at baseline. Data for 188 participants diagnosed with dementia and 267 participants with cerebrovascular disease (e.g., subarachnoid or intracerebral hemorrhage, occlusion or stenosis of the cerebral arteries) at baseline or any follow-up evaluations were excluded. Determination of dementia status is detailed elsewhere (14). Briefly, participants were selected for more comprehensive evaluations based on screening criteria on the Blessed Information Memory and Concentration Scale (BIMCS) (15). Using neuropsychological diagnostic tests and clinical data, diagnoses were made at consensus diagnostic conferences based on Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised criteria for dementia and the National Institute of Neurological and Communication Disorders-Alzheimer’s Disease and Related Disorders Association criteria for Azheimer’s disease (16). Data for 67 individuals not reviewed by case conference at the time of the current study but exhibiting five or more errors on the BIMCS at any administration were excluded from analyses to protect against inclusion of individuals with dementia. The National Institute on Aging Intramural Research Program Institutional Review Board approved this study and all subjects gave written informed consent.
The final total sample comprised 1,586 individuals, although the sample size varied for each cognitive test (see below), ranging from 799 to 1,484 participants. Participants ranged in age from 50.0 to 96.7 years (mean = 65.43, SD = 10.29) at baseline and had on average 16.25 years of education (SD = 2.85). The follow-up interval ranged from 0 to 26 years (mean = 4.40, SD = 4.68). Participant characteristics for the entire sample and for individual cognitive tests are presented in Table 1.
Neuropsychological Assessment
The neuropsychological tests included in the current study assessed five different cognitive domains: 1) learning and memory, 2) attention and executive functions, 3) verbal and language abilities, 4) visuospatial functioning, and 5) general cognitive status. Verbal learning and memory were assessed using the California Verbal Learning Test (CVLT) (17). For this study, analyses of the CVLT focused on overall learning (i.e., total free recall summed across trials 1 through 5 of list A: CVLT-A) and long-term verbal memory (i.e., long-delay free recall: LDFR). Total errors on the Benton Visual Retention Test (BVRT) (18) served as a measure of visual memory. The digit span subtest from the Wechsler Adult Intelligence Scale–Revised (WAIS-R) (19), the Trail Making Test (20) parts A (TMT-A) and B (TMT-B), and verbal fluency (FAS (18) and semantic (21) fluency) assessed attention and executive functions. The Boston Naming Test (BNT) (22) and verbal fluency were administered to assess verbal and language abilities. The Card Rotations Test (23) assessed visuospatial abilities. Finally, the Mini Mental State Exam (MMSE) (24) and BIMCS (15) were measures of general cognitive status.
The cognitive testing program for the BLSA has evolved over the course of the longitudinal study; thus, tests are administered on a time- and age-based schedule. Consequently, not all subjects received all cognitive tests, and subjects may have had a variable number of observations for each test depending on the year and age at entry into the study. In the current study, data for the CVLT, BVRT, digit span, and card rotations tests include participants age 50 and older, while data for participants age 60 and older are included for the TMT, verbal fluency, BNT, BIMCS, and MMSE. Final sample sizes for each individual test are presented in Table 1. Descriptive statistics for the cognitive measures are provided in Table 2.
Depressive Symptomatology
The Center for Epidemiologic Studies Depression Scale (CES-D) (25) provided a measure of depressive symptoms. The CES-D is a widely-used 20-item inventory that assesses the frequency and severity of depressive symptoms experienced in the past week. CES-D scores were used as a continuous variable in all statistical analyses. Separate analyses were performed using concurrent, baseline, and average CES-D scores. The analysis of concurrent CES-D score and cognitive performance included testing sessions in which both the CES-D and the cognitive measure of interest were administered. This analysis provided an estimate of the extent to which cognition is affected by elevated depressive symptoms at the time of cognitive performance. Concurrent CES-D by age interactions would indicate that older individuals are more affected by elevated depressive symptoms at the time of cognitive performance than are younger individuals. For baseline CES-D analyses, participants were included only if the CES-D was administered at the first administration of the neuropsychological test of interest. This approach, which is the method used by most previous studies of depressive symptoms and cognitive decline, examines whether depressive symptoms measured at one time point (baseline) predict subsequent cognitive decline. Baseline CES-D by age interactions would suggest that at older ages, high depressive symptoms at one time point are associated with greater subsequent decrements in cognitive performance. Average CES-D served as a measure of chronic depressive symptoms and was calculated as the average of all available CES-D scores for participants during the study interval, including sessions in which the neuropsychological tests were not administered. Interactions between average CES-D and age may reflect a greater effect of chronic depressive symptoms on cognition at older ages. To provide a more accurate measurement of chronic depressive symptoms, average CES-D analyses were based only on subjects with two or more assessments.
Statistical Analyses
We performed a series of linear mixed models with neuropsychological outcomes as dependent measures using the PROC MIXED procedure in SAS 9.1 (SAS Institute, Cary, NC). This procedure yields information on the unique effects of each predictor, including both fixed and random effects, adjusting for all other terms in the analysis. Mixed-effects models account for correlations among repeated measurements on the same participant and are unaffected by unequal numbers of assessments among individuals. Thus, mixed-effects analyses are the preferred method of examining data with repeated outcome measurements obtained at non-uniform intervals (26).
Separate analyses were conducted using concurrent, baseline, and average CES-D scores to predict each cognitive variable of interest. Baseline age, time interval, and interval2 were additional independent variables. Time interval represents years since baseline testing for each administration of the dependent measure and indexes longitudinal age change, while baseline age indexes cross-sectional age differences. All two-way interactions, as well as three-way interactions of interest, were initially entered into the models. The models included fixed effects of all independent variables and their interactions and random effects of intercept and interval. A backward elimination procedure was employed in which all lower order terms remained in the model while nonsignificant interaction terms (p > 0.05) were eliminated from the model in stages until a final solution was reached (27). Sex, self-reported race, education, and scores on the Primary Mental Abilities vocabulary test (PMA; 28) were entered as covariates. Race × education was also entered as a covariate because it was significantly associated with CES-D scores. Antidepressant use was initially entered as a covariate but was subsequently removed from the models because it was a nonsignificant predictor in all analyses. All variables were treated as continuous variables, with the exception of sex (0 = women, 1 = men) and race (0 = white, 1 = nonwhite), which were treated as class variables. Results are reported without correction for multiple comparisons to avoid type II errors (29). Rather, effect sizes are presented as a measure of the magnitude of the effects (30). Effect sizes were estimated with Cohen’s d applied to covariate adjusted means. Change scores were used in the effect size calculation for interval effects. Graphing of individual models, performed using SigmaPlot 10 for Windows, reflects estimated scores computed from parameter estimates of the individual mixed-effects regression equations including all main effects and significant interactions. Although depressive symptoms were treated as continuous variables in all analyses, for ease of visual display graphs depict two depression groups using a cutoff of 16 on the CES-D, a well-accepted, standard cutoff for identification of clinically significant depressive symptoms (31).
Results
The results of mixed-effects regression analyses, adjusted for sex, education, race, and PMA Vocabulary scores, are shown in Table 3 – 5. Results for tests which showed significant CES-D effects are graphically shown in Figure 1 –3. Overall, the models showed expected cross-sectional effects of baseline age on cognition. Significant interval effects, reflecting longitudinal decline, were also observed for most measures, and interval2 effects for some measures revealed accelerating longitudinal decline during the follow-up period. With the exception of digits forward, letter fluency, and the MMSE, all measures showed significant baseline age × interval effects in which younger age at baseline was associated with stable or slightly improved performance over time while older baseline age was associated with longitudinal cognitive decline. This effect suggests that younger individuals experienced a practice effect, but practice effects were overwhelmed by cognitive deterioration in older individuals.
Figure 1.
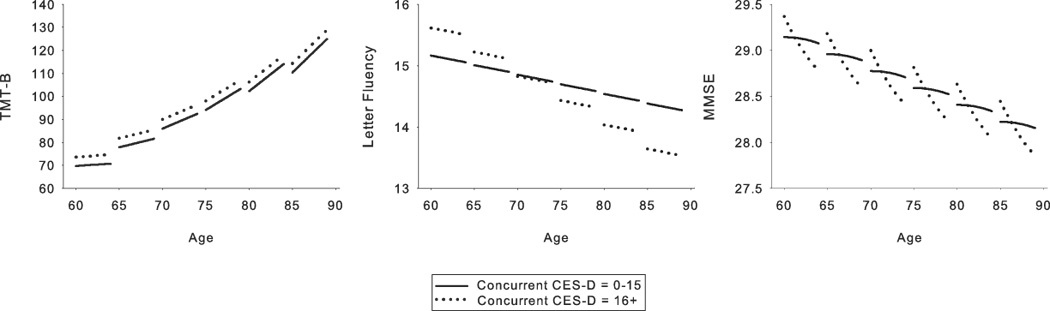
Relationship between concurrent depressive symptoms and cognition for those measures that showed significant concurrent depressive symptoms effects. Each line segment represents estimated values for 4 years of longitudinal data. Solid line = Center for Epidemiologic Studies Depression Scale (CES-D) scores of 0–15; dotted line = CES-D scores of 16 and greater. The depressive symptoms groupings depicted in the figure are for ease of display only as depressive symptoms were a continuous variable in all analyses. Parallel slopes for the two depressive symptoms groups in the Trailmaking Test, part B (TMT-B) and Letter Fluency graphs indicate that there were cross-sectional effects but no significant differences in longitudinal cognitive decline across the range of depressive symptoms. In the graph for the Mini Mental State Exam, the steeper slope of the line representing CES-D scores of 16 or greater reflects greater longitudinal decline as a function of higher depressive symptoms.
Figure 3.
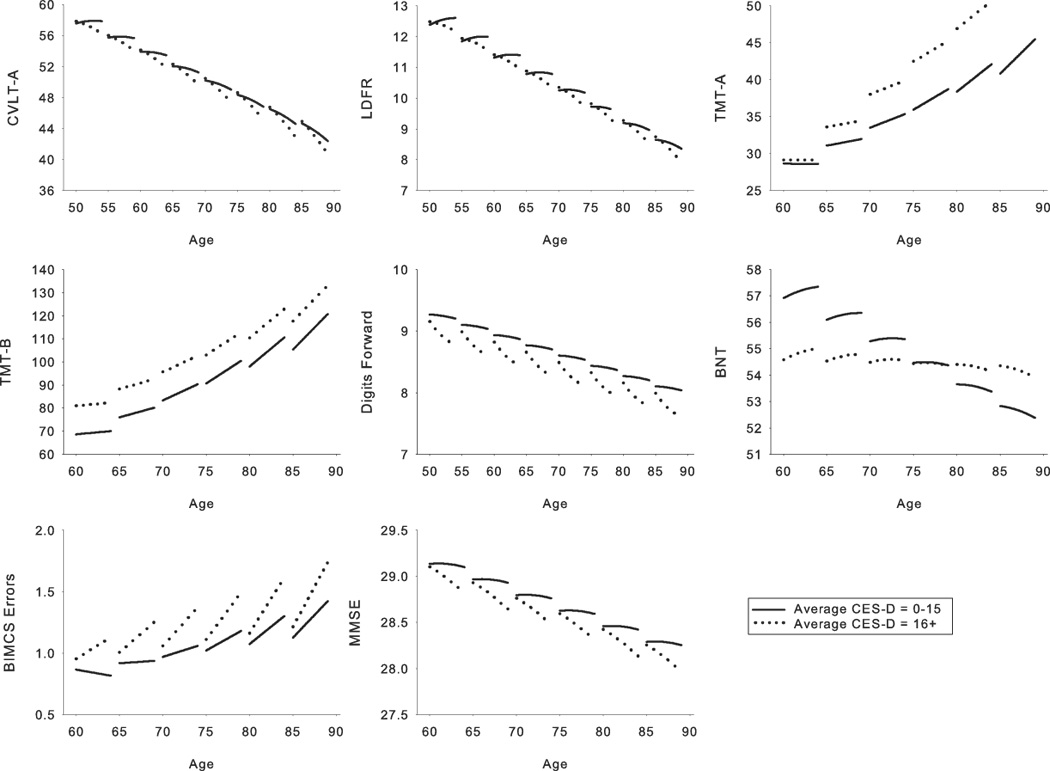
Relationship between average depressive symptoms and cognition for those measures that showed significant average depressive symptoms effects. Each line segment represents estimated values for 4 years of longitudinal data. Solid line = Center for Epidemiologic Studies Dotson Depression Scale (CES-D) scores of 0–15; dotted line = CES-D scores of 16 and greater. The depressive symptoms groupings depicted in the figure are for ease of display only as depressive symptoms were a continuous variable in all analyses. Parallel slopes for the two depressive symptoms groups in the Trailmaking Test, parts A (TMT-A) and B (TMT-B) and the Boston Naming Test (BNT) graphs indicate that there were cross-sectional effects but no significant differences in longitudinal cognitive decline across the range of depressive symptoms. The steeper slope of the line representing CES-D scores of 16 or greater for the California Verbal Learning Test List A (CVLT-A) and Long Delay Free Recall (LDFR), digits forward, Blessed Information Memory and Concentration Scale (BIMCS), and the Mini Mental State Exam (MMSE) graphs reflects greater longitudinal decline as a function of higher depressive symptoms.
Concurrent depressive symptoms
Results for concurrent depressive symptoms analyses are presented in Table 3 and Figure 1. A cross-sectional effect of depressive symptoms on TMT-B [F(1, 1495) = 4.49, p = .028] and letter fluency [F(1, 1538) = 4.88, p = .039] was reflected in a significant CES-D effect in concurrent depressive symptoms analyses. For letter fluency, a baseline age × CES-D effect [F(1, 1538) = 4.71, p = .043] revealed an adverse impact of depressive symptoms for older (age 75+), but not younger individuals. Significant CES-D × interval [F(1, 1529) = 10.01, p = .002] and CES-D × interval2 [F(1, 1529) = 10.08, p = .002] effects for the MMSE indicated that increased depressive symptoms were associated with a steeper slope of decline in general cognitive status, and this effect accelerated over time. Effect sizes ranged from .24 to −.28 for significant cross-sectional effects and from −1.36 to −1.91 for significant longitudinal effects.
Baseline depressive symptoms
Results for baseline depressive symptoms analyses are presented in Table 4 and Figure 2. Increased depressive symptoms at baseline predicted greater longitudinal decline on digits forward [F(1, 2252) = 6.40, p = .031] and the MMSE [F(1, 1610) = 5.42, p = .021], as indicated by significant CES-D × interval effects. Significant baseline CES-D × interval2 effects for these measures [digits forward F(1, 2252) = 5.13, p = .039; MMSE F(1, 1610) = 4.17, p = .043] revealed accelerating longitudinal decline over the course of the follow-up period for individuals with more depressive symptoms at baseline. Effect sizes ranged from −1.91 to −1.94.
Figure 2.
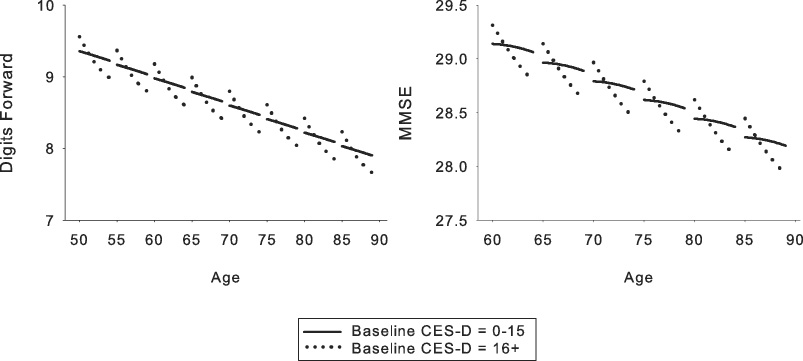
Relationship between baseline depressive symptoms and cognition for those measures that showed significant baseline depressive symptoms effects. Each line segment represents estimated values for 4 years of longitudinal data. Solid line = Center for Epidemiologic Studies Depression Scale (CES-D) scores of 0–15; dotted line = CES-D scores of 16 and greater. The depressive symptoms groupings depicted in the figure are for ease of display only as depressive symptoms were a continuous variable in all analyses. The steeper slope of the line representing CES-D scores of 16 or greater in both graphs reflects greater longitudinal decline as a function of higher depressive symptoms.
Average depressive symptoms
Results for average depressive symptoms analyses are presented in Table 4 and Figure 3. Higher average depressive symptoms were associated with poorer performance on TMT-A [F(1, 610) = 4.96, p = .028] and TMT-B [F(1, 609) = 11.23, p = .002]. For TMT-A, the average CES-D effect increased as a function of baseline age [F(1, 610) = 6.86, p = .009]. Effect sizes for the TMT ranged from .73 to .74. Average CES-D [F(1, 604) = 8.51, p = .004, effect size = −.36] and baseline age × average CES-D [F(1, 604) = 7.17, p = .007, effect size = 2.04] effects were found for the BNT, reflecting cross-sectional age effects for lower, but not higher depressive symptoms. Individuals with higher average CES-D scores showed greater longitudinal decline on CVLT-A [F(1, 1843) = 5.03, p = .031], LDFR [F(1, 1843) = 6.27, p = .029], the BIMCS [F(1, 1778) = 3.88, p = .013], and the MMSE [F(1, 1749) = 4.97, p = .031], with effect sizes ranging from 1.33 to −1.91. An average CES-D × interval2 interaction for digits forward revealed accelerating longitudinal decline as a function of average depressive symptoms [F(1, 2315) = 4.51, p = .042].
Conclusions
In this longitudinal study of community-dwelling dementia-free older adults, we examined the relationship between depressive symptoms and cognitive function and decline in as many as 19 repeated assessments over as much as a 26-year follow-up. Depressive symptoms were associated with poor cognitive functioning and longitudinal cognitive decline in multiple domains. Analysis of concurrent depressive symptoms and cognitive performance provided a measure of the cross-sectional impact of depressive symptoms on cognitive functioning. Depressive symptoms were found to have a significant cross-sectional effect on executive functions and general cognitive status. Longitudinal analyses revealed that depressive symptoms at baseline predicted longitudinal decline in attention and general cognitive status. Moreover, higher average depressive symptoms, which may reflect chronic or persistent symptoms, were associated with executive dysfunction as well as longitudinal decline in memory, attention, and general cognitive status. Thus, concurrent, baseline, and average depressive symptoms had differential associations with cognitive decline. Average depressive symptoms appeared to show the most extensive effects on cognition, although our three sets of analyses were not formally contrasted. Taken together, our results suggest that depressive symptoms have widespread effects on cognitive function and decline. In conjunction with previous findings that persistent, but not episodic, depressive symptoms are associated with cognitive decline (4), our results suggest that prolonged depressive symptoms may have a greater impact on cognition than transient symptoms.
The age by depressive symptoms interactions observed for some executive measures are consistent with previous findings (3) and suggest that older individuals are more vulnerable to the adverse impact of depressive symptoms on cognition, at least for some cognitive functions. However, it should be noted that this effect was limited to two measures. Furthermore, advanced age was associated with a greater effect of depressive symptoms on cognitive functioning (i.e., cross-sectional effect), but not on cognitive decline (i.e., longitudinal effect).
Our results are consistent with a growing number of investigations reporting an association between depressive symptoms and subsequent cognitive decline (7−9). However, findings in the literature are mixed, as some investigators have not observed such a relationship (2, 10). These inconsistent findings are attributable to a number of methodological differences between studies, including participant characteristics, definitions of depressive symptoms and cognitive decline, and the use of continuous versus categorical variables. In addition, many studies limit analyses to one or two cognitive domains. Our study offers the advantage of a relatively large sample size, a long follow-up interval with prospective assessments, and the use of continuous measures of cognition and depressive symptoms, which provides a more sensitive assessment of significant relationships. In addition, we assessed functioning in a variety of cognitive domains, allowing for distinctions to be made between abilities which are differentially affected by age and depression. Moreover, our study is unique in its examination of concurrent depressive symptoms, depressive symptoms at baseline, as well as average depressive symptoms. Such examination allows us to provide some clarification about the longitudinal relationship between depressive symptoms, cognitive functioning and decline, and aging.
There are several hypotheses that might explain the direction of the relationship between depression and cognitive decline (see meta-analysis by Jorm (32) for a review). Depression may precede cognitive deficits, which would suggest that depression is a prodrome of or risk factor for cognitive decline. Indeed, a number of studies have shown that a history of depression is a strong risk factor for dementia, a finding that was further supported by a recent meta-analysis (33). Some authors have attributed this risk to hippocampal damage caused by depression pathology, such as increased glucocorticoid secretion (34). Others have suggested that late-onset depression, in particular, is a prodrome of dementia. A recent finding that the interval between the diagnoses of depression and Alzheimer’s disease (AD) is positively related to an increased risk of developing AD (33) suggests that depression may be a risk factor, rather than a prodrome, for AD.
It has also been postulated that depression is a psychological reaction to perceived cognitive loss in older adults. Some studies have found that cognitive impairment precedes the onset of depressive symptoms, while depressive symptoms do not predict accelerated cognitive decline (35). These results were interpreted as evidence that the awareness of cognitive decline led to depression as a psychological reaction to the loss of cognitive functioning. However, there is evidence that declines in function, but not in cognition, precede the first episode of depressive symptoms in patients with probable AD (36). In addition, depressive symptoms that precede cognitive decline may be predominately motivation-related (e.g., lack of interest, loss of energy, and concentration difficulties), not mood-related (37), which suggests that these symptoms represent disease-related changes in the brain, rather than reaction to self-perceived cognitive changes.
An alternative explanation of the relationship between depression and cognitive decline is that both conditions are a result of common risk factors or underlying neurodegenerative process. Both aging and depression are associated with frontal pathology (38, 39). In addition, disruption of frontal-subcortical circuits, neurotransmitter changes, vascular changes, and genetic links are common to both aging and depression (40–43). This evidence suggests that common neurobiological and genetic factors may contribute to the development of both cognitive difficulties and depressive symptoms.
It is possible that more than one of these hypotheses explain the relationship between cognition and depression. We investigated depressive symptoms rather than clinical depression and cannot distinguish between possible explanations of reported associations between clinical depression and cognitive decline. Information about the presence of psychiatric conditions was not available for our participants, thus, our inability to rule out the possibility that the presence of other psychiatric conditions influenced our results is a limitation of this study. The lack of information regarding the age at onset of depressive symptoms is an additional limitation of our study, as early and late onset depression may have different etiological contributors. Additionally, information about functional outcomes (e.g., performance of instrumental activities of daily living) would have been useful in establishing the clinical relevance of the observed effects; however, this information was not available for our sample. Furthermore, our sample is not population based, and most participants are highly educated, white, and male, limiting the generalizability of our findings. However, the relative homogeneity of the sample may also be seen as a strength of this study because it diminishes possible effects of demographic variables as confounds. Additionally, our results are consistent with a previous study in which baseline depressive symptoms predicted cognitive decline over seven years in a primarily white, high functioning sample (9). Finally, given the number of statistical analyses performed, the inflated type I error rate is a limitation of the study and suggests that statistically less pronounced differences should be interpreted with caution. However, given the novel design of our study, which examined the effect of concurrent, baseline, and average depressive symptoms on cognitive functioning and decline in older adults, we were willing to tolerate a higher chance of type I error rather than miss a true positive finding (29). Moreover, the large effect sizes observed for the majority of our models would suggest that the differences are indeed meaningful rather than spurious findings (30). These limitations should be viewed in the context of the strengths of the study, such as the large sample size, relatively long follow-up period with prospective assessments, and well characterized nature of the sample. Our findings are important because they provide additional evidence from a well characterized sample that subthreshold depressive symptoms are associated with poor cognitive functioning and longitudinal cognitive decline.
Recently, there has been increasing recognition that subthreshold depressive symptoms are associated with important clinical outcomes in older adults, including medical burden and functional status (44–47). In light of the reported association between cognitive deficits and functional impairment in geriatric depression (48), our findings suggest that subthreshold depressive symptoms may also have clinical significance, although we did not assess functional abilities in the current study. The finding that, compared to concurrent and baseline depressive symptoms, average depressive symptoms were associated with cognitive dysfunction on the most cognitive measures highlights the importance of how depressive symptoms are measured in investigations of the association between cognition and depression. It also suggests that clinicians should consider the chronicity of depressive symptoms when evaluating cognitive functioning in older adults (e.g., by inquiring about the duration of symptoms beyond the 1–2 week criteria used for most assessment instruments) and that a single concurrent assessment of depressive symptoms may not be a good predictor of future cognitive status. Determining the temporal relationship between cognitive decline and depression or depressive symptoms in late life is an important area for further examination. Future studies that explore underlying neural changes and that examine the effect of subtypes of clinical depression on cognition will further elucidate the relationship between depressive symptoms and cognitive decline in late life.
Acknowledgments
The National Institute on Aging Intramural Research Program of the National Institutes of Health supported this research.
References
- 1.Boone KB, Lesser I, Miller B, et al. Cognitive functioning in a mildly to moderately depressed geriatric sample: relationship to chronological age. J Neuropsychiatry Clin Neurosci. 1994;6(3):267–272. doi: 10.1176/jnp.6.3.267. [DOI] [PubMed] [Google Scholar]
- 2.Comijs HC, Jonker C, Beekman AT, et al. The association between depressive symptoms and cognitive decline in community-dwelling elderly persons. Int J Geriatr Psychiatry. 2001;16(4):361–367. doi: 10.1002/gps.343. [DOI] [PubMed] [Google Scholar]
- 3.Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159(7):1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- 4.Paterniti S, Verdier-Taillefer MH, Dufouil C, et al. Depressive symptoms and cognitive decline in elderly people. Longitudinal study. Br J Psychiatry. 2002;181:406–410. doi: 10.1192/bjp.181.5.406. [DOI] [PubMed] [Google Scholar]
- 5.Ravdin LD, Katzen HL, Agrawal P, et al. Letter and semantic fluency in older adults: effects of mild depressive symptoms and age-stratified normative data. Clin Neuropsychol. 2003;17(2):195–202. doi: 10.1076/clin.17.2.195.16500. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Wilson RS, Schneider JA, et al. Cerebral infarctions and the relationship of depression symptoms to level of cognitive functioning in older persons. Am J Geriatr Psychiatry. 2004;12(2):211–219. [PubMed] [Google Scholar]
- 7.Sachs-Ericsson N, Joiner T, Plant EA, et al. The influence of depression on cognitive decline in community-dwelling elderly persons. Am J Geriatr Psychiatry. 2005;13(5):402–408. doi: 10.1176/appi.ajgp.13.5.402. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Mendes De Leon CF, Bennett DA, et al. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. 2004;75(1):126–129. [PMC free article] [PubMed] [Google Scholar]
- 9.Chodosh J, Kado DM, Seeman TE, Karlamangla AS. Depressive symptoms as a predictor of cognitive decline: MacArthur Studies of Successful Aging. Am J Geriatr Psychiatry. 2007;15(5):406–415. doi: 10.1097/01.JGP.0b013e31802c0c63. [DOI] [PubMed] [Google Scholar]
- 10.Vinkers DJ, Gussekloo J, Stek ML, et al. Temporal relation between depression and cognitive impairment in old age: prospective population based study. BMJ. 2004;329(7471):881. doi: 10.1136/bmj.38216.604664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassuk SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Arch Gen Psychiatry. 1998;55(12):1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- 12.Geerlings MI, Schoevers RA, Beekman AT, et al. Depression and risk of cognitive decline and Alzheimer's disease. Results of two prospective community-based studies in The Netherlands. Br J Psychiatry. 2000;176:568–575. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- 13.Shock NW, Greulich RC, Andres R, et al. Normal human aging: the Baltimore longitudinal study of aging. Washington, DC: U.S. Government Printing Office; 1984. [Google Scholar]
- 14.Kawas C, Gray S, Brookmeyer R, et al. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54(11):2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 15.Blessed G, Wilson ID. The contemporary natural history of mental disorder in old age. Br J Psychiatry. 1982;141:59–67. doi: 10.1192/bjp.141.1.59. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Delis D, Kramer J, Kaplan E, et al. California Verbal Learning Test: Adult version. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 18.Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6(1):53–60. [Google Scholar]
- 19.Wechsler D. Wechsler Adult Intelligence Scale—Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 20.Reitan R. Trail Making Test: Manual for Administration and Scoring. Tucson, AZ: Reitan Neuropsychological Laboratory; 1992. [Google Scholar]
- 21.Newcombe F. Missile wounds of the brain: A study of psychological deficits. London: Oxford University Press; 1969. [Google Scholar]
- 22.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1978. [Google Scholar]
- 23.Wilson J, Vandenberg S. In: Sex differences in cognition: Evidence from the Hawaii Family Study, in Sex and Behavior. McGill T, Dewsbury D, Sachs B, editors. New York: Plenum; 1978. pp. 317–335. [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 26.Gueorguieva R, Krystal JH. Move over ANOVA: Progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 27.Morrell CH, Pearson JD, Brant LJ. Linear Transformations of Linear Mixed-Effects Models. Am Stat. 1997;51(4):338–343. [Google Scholar]
- 28.DeFries JC, Vandenberg SG, McClearn GE, et al. Near identity of cognitive structure in two ethnic groups. Science. 1974;183(122):338–339. doi: 10.1126/science.183.4122.338. [DOI] [PubMed] [Google Scholar]
- 29.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garamszegi LZ. Comparing effect sizes across variables: generalization without the need for Bonferroni correction. Behav Ecol. 2006;17(4):682–687. [Google Scholar]
- 31.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 32.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35(6):776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 33.Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sapolsky RM. Why stress is bad for your brain. Science. 1996;273(5276):749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 35.Chen P, Ganguli M, Mulsant BH, et al. The temporal relationship between depressive symptoms and dementia: a community-based prospective study. Arch Gen Psychiatry. 1999;56(3):261–266. doi: 10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- 36.Holtzer R, Scarmeas N, Wegesin DJ, et al. Depressive symptoms in Alzheimer's disease: natural course and temporal relation to function and cognitive status. J Am Geriatr Soc. 2005;53(12):2083–2089. doi: 10.1111/j.1532-5415.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 37.Berger AK, Fratiglioni L, Forsell Y, et al. The occurrence of depressive symptoms in the preclinical phase of AD: a population-based study. Neurology. 1999;53(9):1998–2002. doi: 10.1212/wnl.53.9.1998. [DOI] [PubMed] [Google Scholar]
- 38.Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- 39.Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, Mahwah TA, editors. The Handbook of Aging and Cognition. 2nd edition. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. pp. 1–90. [Google Scholar]
- 40.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60(12):1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Goldman-Rakic PS, Brown RM. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience. 1981;6(2):177–187. doi: 10.1016/0306-4522(81)90053-1. [DOI] [PubMed] [Google Scholar]
- 42.Klimek V, Schenck JE, Han H, et al. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiatry. 2002;52(7):740–748. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- 43.Tsai SJ, Hong CJ, Liu HC, et al. Association analysis of brain-derived neurotrophic factor Val66Met polymorphisms with Alzheimer's disease and age of onset. Neuropsychobiology. 2004;49(1):10–12. doi: 10.1159/000075332. [DOI] [PubMed] [Google Scholar]
- 44.Brodaty H, Withall A, Altendorf A, Sachdev PS. Rates of Depression at 3 and 15 Months Poststroke and Their Relationship With Cognitive Decline: the Sydney Stroke Study. Am J Geriatr Psychiatry. 2007;15(6):477–486. doi: 10.1097/JGP.0b013e3180590bca. [DOI] [PubMed] [Google Scholar]
- 45.Chopra MP, Zubritsky C, Knott K, Have TT, Hadley T, Coyne JC, Oslin DW. Importance of subsyndromal symptoms of depression in elderly patients. Am J Geriatr Psychiatry. 2005;13(7):597–606. doi: 10.1176/appi.ajgp.13.7.597. [DOI] [PubMed] [Google Scholar]
- 46.Lyness JM, Kim J, Tang W, Tu X, Conwell Y, King DA, Caine ED. The clinical significance of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry. 2007;15(3):214–223. doi: 10.1097/01.JGP.0000235763.50230.83. [DOI] [PubMed] [Google Scholar]
- 47.Sachs-Ericsson N, Burns AB, Gordon KH, Eckel LA, Wonderlich SA, Crosby RD, Blazer DG. Body Mass Index and Depressive Symptoms in Older Adults: The Moderating Roles of Race, Sex, and Socioeconomic Status. Am J Geriatr Psychiatry. 2007;15(9):815–825. doi: 10.1097/JGP.0b013e3180a725d6. [DOI] [PubMed] [Google Scholar]
- 48.Kiosses DN, Alexopoulos GS. IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr Psychiatry. 2005;13(3):244–249. doi: 10.1176/appi.ajgp.13.3.244. [DOI] [PubMed] [Google Scholar]


