Abstract
Objective
This study examined the relationship between quality of dietary carbohydrate intake, as measured by glycemic index (GI) and glycemic load (GL), and high sensitivity serum C-reactive protein (hs-CRP) levels.
Method
During a one-year observational study, data were collected at baseline and at each quarter thereafter. GI and GL were calculated from multiple 24-hour dietary recalls (24HRs), three randomly selected 24HRs at every quarter, with up to fifteen 24HRs per participant. Hs-CRP was measured in blood samples collected at baseline and each of the four quarterly measurement points. Multivariable linear mixed models were used to examine both the cross-sectional and the longitudinal association of GI, GL, and hs-CRP.
Results
Among 582 adult men and women with at least two measures of both diet and hs-CRP, average daily GI score (white bread=100) was 85 and average GL was 198, Average hs-CRP was 1.84 mg/l. Overall, there was no association between either GI or GL and hs-CRP. Subgroup analyses revealed an inverse association between GL and hs-CRP, among obese individuals (body mass index ≥30 kg/m2).
Conclusions
Quality of dietary carbohydrates does not appear to be associated with serum hs-CRP levels. Among obese individuals higher dietary GL appears to be related to lower hs-CRP levels. Due to the limited number of studies on this topic and their conflicting results, further investigation is warranted.
Keywords: glycemic index, glycemic load, high-sensitivity-CRP, diet, carbohydrate, cardiovascular diseases
Introduction
Cardiovascular disease and diabetes are two leading causes of morbidity and mortality in the U.S. and worldwide. According to the CDC in 2005 it was estimated that 25.6 million non-institutionalized Americans have some form of heart disease and over 20 million have diabetes [1,2]. Among those with diabetes, 65% will die from heart disease or stroke [3].
High sensitivity C-reactive protein (hs-CRP), a marker of inflammation, has been recognized as a risk factor for future cardiac events [4-12]. While very high hs-CRP levels are likely the response to acute inflammation, slightly elevated levels are indicative of chronic inflammation present in such diseases as cardiovascular, and diabetes. Hs-CRP values are useful in determining disease progression or the effectiveness of treatments, and since many of these diseases are modifiable by lifestyle, tracking hs-CRP can be quite informative. It is important to identify which lifestyle factors have the greatest impact, since lifestyle factors may provide an important intervention opportunity to beneficially influence hs-CRP levels in order to reduce the risk of cardiovascular disease and diabetes.
Diet is one of the many modifiable risk factors for both cardiovascular disease and diabetes. There is increasing evidence that both quantity and quality of carbohydrate can modify disease risk [13,14]. One method to evaluate the quality of carbohydrate is the glycemic index (GI); a measure of the blood glucose response to 50 grams of carbohydrate from a particular food [15]. Glycemic load (GL) is the GI of a food multiplied by its carbohydrate content in grams (quality times quantity). The present study examined the relationship between GI and GL and, hs-CRP, a marker of inflammation, among a population of healthy adults.
Participants and Methods
Data for this study was obtained from a one-year prospective observational study designed to examine seasonal variations in blood lipid levels in a disease free population in central Massachusetts [16]. The study began in 1996 with 641 eligible participants enrolled at baseline. Eligibility requirements include age between 20 and 70 years, literate in English, and not planning to leave the area within the next year [16]. Exclusion criteria included: 1) using or planning to use lipid lowering drugs; 2) following or planning to follow a weight control diet; 3) a history of cancer diagnosis (excluding nonmelanoma) within the past 5 years; 4) a secondary cause of hyperlipidemia; or 6) a condition of psychiatric illness that would limit participation [16]. Data collection took place at baseline and quarterly (every 13 weeks) thereafter for a total of five visits. Each visit included measurements of anthropometrics, blood, diet, physical activity, and psychosocial variables.
High sensitivity CRP Assessment
Participants were evaluated quarterly for a total of five visits over the one year follow-up. At each of their five clinic visits, participants provided blood samples. Hs-CRP levels were measured at Children's Hospital in Boston at Dr. Nader Rifai's laboratory using latex-enhanced immunonephelometric assays on a BN II analyzer.
Dietary Assessment
Trained registered dietitians assessed diet via telephone based 24 hour recalls (24HRs), using the Minnesota Nutrition Data System (NDS DOS, versions 2.6, 2.7 and 2.8) developed by the Nutrition Coordinating Center (NCC) at the University of Minnesota. Three telephone interviews occurred at baseline and at four additional data collection periods, within a window of two weeks prior to three weeks after the participants' quarterly clinic visits. Therefore, up to 15 days of dietary information was available per participant including both weekdays and weekends, allowing for a comprehensive measure of usual carbohydrate intake [18].
Methods of GI Resolution
The quality of carbohydrate can be determined by the GI, which ranks foods according to their effect on blood glucose [15]. Although not all carbohydrate containing foods have been tested for their GI, the GI of over 1,500 foods has been determined and are available in the International Table of Glycemic Index and Glycemic Load Values with additional foods continually added to an online database (15, 19,20]. We assigned GI and GL values to foods reported on the 24HR using methods described previously [21]. Briefly, carbohydrate-containing foods derived from 24HRs were matched to the International Table of Glycemic Index and Glycemic Load Values. Mixed foods were disaggregated into individual ingredients. For specific foods not found in the table, we based estimates on similar foods according to physical and chemical factors that determine GI.
The GL was calculated to take into account the amount of carbohydrate that was consumed. GL equals the GI of a food times the amount of carbohydrate eaten divided by 100. For example, let's consider ½ cup (37 g) of potatoes. The GI of potatoes is 102 (white bread as reference=100). Using the formula, the GL for this portion is, 102 X 37g/100= 38.
Covariate Assessment
A number of factors have consistently been associated with hs-CRP [22,23]. We collected data on BMI, smoking, infection status, physical activity, total energy, total cholesterol, age and gender, and included these variables in analyses.
Statistical Analysis
Means and standard deviation were used to describe characteristics of participants, their hs-CRP, GI, and GL values. Scatter plot and smoothing curves (i.e., lowess curves) were used to explore graphically the relationship of hs-CRP with GL, and GI.
To make use of the rich dataset we used a mixed model which examines both the cross-sectional (between -subjects) association of CRP with glycemic load and the longitudinal association (within-subject). We used all data in the analyses. The dependent variable for this analysis was CRP. Independent variables were GI or GL, with subject treated as random effect. Specifically, the cross-sectional effects for a variable (GI or GL) were assessed by including a variable representing the subject mean; the longitudinal effects were assessed by including a variable representing the deviation from the subject mean from each quarter. Thus, the cross sectional analysis examined the association between the average CRP and average GI or GL among the subjects at each quarter. The longitudinal analysis compared individual changes in CRP and GI or GL between the quarters. Both the cross-sectional and longitudinal effects were included in the same model. This method has been used in our previous analyses of the association between dietary fiber and CRP [27]. Covariates that were significantly associated with both the GI or GL and hs-CRP in the bivariate analysis were included in the final model. If a covariate changed either exposure coefficient by 15% or more and was statistically significant at p= 0.15, it was included in the final model. When either calorie or fat intake was included in the model, it was not significant; the regression coefficient of hs-CRP with GI or GL was not changed, so neither calories nor fat intake were included in the final model. When we included season of year as a covariate, results were not changed, so it was not included in the final model. We assessed for effect modification by BMI category and gender.
Results
A total of 641 participants with 2,795 observations were available for analysis. Because our goal was to examine the longitudinal effect of dietary GI or GL on hs-CRP, participants with data available for less than 2 times point were excluded (n=49 observations). An additional 617 observations were excluded because both dietary measurements and hs-CRP were not available at the same quarter. We excluded 65 observations where hs-CRP was greater than 10 mg/L because such elevated levels are likely to be caused by an acute infection or underlying medical problem not related to diet [24]. We also excluded one observation with an extreme outlier; GL= 1085. Therefore a total of 582 participants and 2063 observations remained for analysis. Hs-CRP values were highly skewed, therefore, the data were analyzed using log transformed values for hs-CRP.
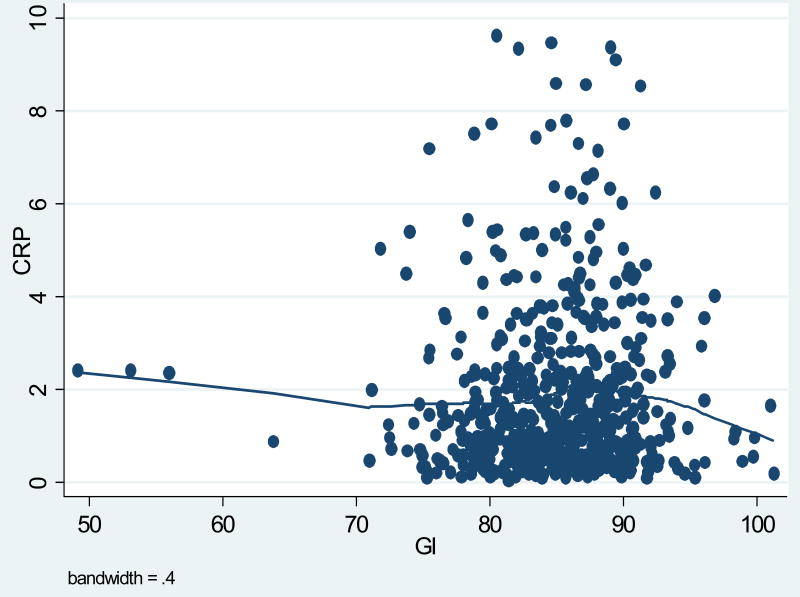
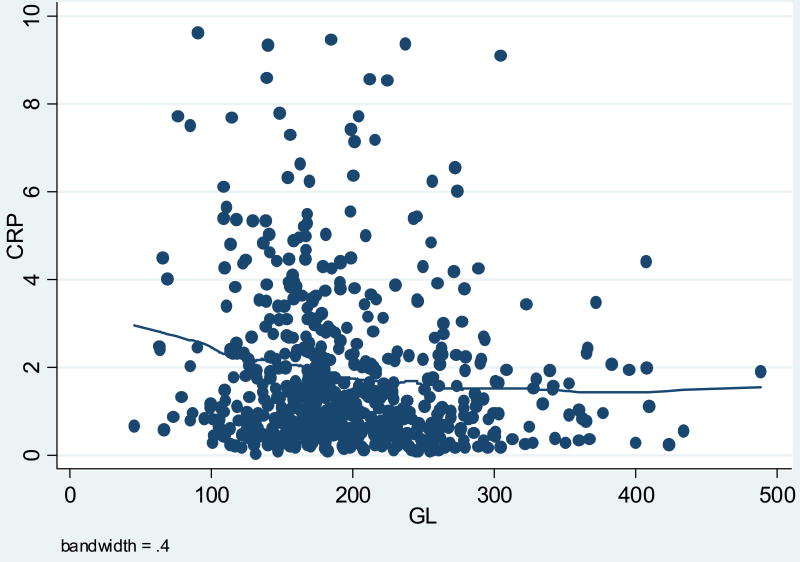
Table 1 presents participant characteristics. Participants were predominantly white (86%), with an average age of 48 years and approximately equal distributions of males and females. Average BMI was 27.4 kg/m2, and 64% of the participants were either overweight or obese. Average and median hs-CRP values were, 1.84 mg/L and 1.21 mg/L, respectively (Table 2). For this paper GI is reported using white bread as the reference, where white bread equals a GI score of 100. The average daily dietary GI score was 85, considered to be in the intermediate range for GI, with values ranging from 49 to 101 (Table 2). The average GL value was 198, considered to be in the high range for GL, with a minimum of 45 and a maximum value of 489 (Table 2). All-purpose flour, white sugar, white bread, white rice and cola beverage, were the top contributors to GL these five foods alone account for a cumulative GL of 52. The graphs in figures one and two suggested a slightly inverse relationship between average GI or GL and hs-CRP levels (Figures 1, 2).
Table 1.
Selected characteristics of the study population.
| Variable | |
|---|---|
| Age (years) | 48 (12) |
| Mean (SD) | |
| BMI (kg/m2) | 27.4 (5.4) |
| Mean (SD) | |
| Race | |
| White (%) | 86 |
| Hispanic White (%) | 8.1 |
| Asian (%) | 1.6 |
| Black (%) | 2.7 |
| Gender | |
| Male (%) | 52 |
| Female (%) | 48 |
Table 2.
Mean median and range of average high sensitivity CRP, average dietary glycemic index and average dietary glycemic load.
| Characteristic | N | Mean
(SD) |
Median | Minimum | Maximum |
|---|---|---|---|---|---|
| hs-CRP
(mg/L) |
582 | 1.8 | 1.2 | 0.03 | 9.6 |
| Log HS-CRP | 582 | 0.06 | 0.08 | 3.73 | 2.27 |
| Glycemic Index | 582 | 84.9 | 85.1 | 49.1 | 101.2 |
| Glycemic Load | 582 | 198.0 | 184.5 | 45.4 | 488.2 |
Figure 1. Scatterplot between Average CRP and Average GI.
Figure 2. Scatterplot between Average CRP and Average GL.
We used a multivariable linear mixed model to examine both the cross-sectional and longitudinal relationship between log hs-CRP and GI or GL (Table 3). We found no association between GI and log hs-CRP in either the cross-sectional (regression coefficient (β)=0.009, p=0.24) or the longitudinal analysis (β=-0.002, p= 0.39). We did however observe the suggestion of an inverse association between GL and log hs-CRP in the cross-sectional analyses, but no association in the longitudinal analyses. Specifically, the coefficient for the cross-sectional effect of GL (β= -0.00194) was suggestive of an inverse relationship with log hs-CRP (p= 0.002).
Table 3.
Regression coefficients predicting log high sensitivity CRP from linear mixed models.
| Cross-Sectional Within Participant | Longitudinal Between Participants | |||||
|---|---|---|---|---|---|---|
| Regression coefficient | SE | P-value | Regression coefficient | SE | P-value | |
| Glycemic Index | 0.008781 | 0.007439 | 0.2380 | -0.00237 | 0.002743 | 0.3881 |
| Glycemic Load | -0.00194 | 0.000622 | 0.0019 | -0.00005 | 0.000307 | 0.8689 |
After statistically adjusting for BMI, smoking status, age and infection status, the cross-sectional findings for GL and hs-CRP are attenuated and no longer statistically significant (p=0.07). The longitudinal association was attenuated to -0.00012 (p= 0.72) (Table 4). None of the variables produced notable changes in the strength or direction of the estimate.
Table 4.
Regression coefficients predicting log high sensitivity CRP from multivariable linear mixed models.
| Cross-Sectional Within Participant | Longitudinal Between Participants | |||||
|---|---|---|---|---|---|---|
| Regression coefficient | SE | P-value | Regression coefficient | SE | P-value | |
| Glycemic Index* | 0.002671 | 0.006269 | 0.6702 | -0.00396 | 0.002832 | 0.1627 |
| Glycemic Load** | -0.00096 | 0.000528 | 0.0683 | -0.00012 | 0.000331 | 0.7197 |
Multivariate model includes BMI, smoking status and, age
Multivariate model includes: BMI, smoking status, age, and infection status
We then stratified the analysis by BMI category and gender. Stratification by gender did not yield results of statistical significance (for cross-sectional results for GL; men β=-0.0055 p=0.43, women β= -0.00141 p=0.18). When stratified by BMI, we found that mean GL was a significant predictor of hs-CRP in the cross-sectional analysis only among obese individuals (β= -0.00185 p=0.04) (Table 5).
Table 5.
Regression coefficients predicting log high sensitivity CRP from multivariable linear mixed models for glycemic load stratified by BMI categories.
| Cross-Sectional Within Participant | Longitudinal Between Participants | |||||
|---|---|---|---|---|---|---|
| BMI category (kg/m2) | Regression coefficient | SE | P-value | Regression coefficient | SE | P-value |
| 18.5 to 24.9 Normal | -0.00100 | 0.000888 | 0.2595 | -0.00070 | 0.000658 | 0.2907 |
| 25.0 to 29.9 Overweight | -0.00063 | 0.000929 | 0.4966 | -0.00022 | 0.000472 | 0.6449 |
| > 30 Obese | -0.00185 | 0.000915 | 0.0440 | 0.000710 | 0.000484 | 0.1433 |
Multivariate model includes: BMI, smoking status, age, and infection status
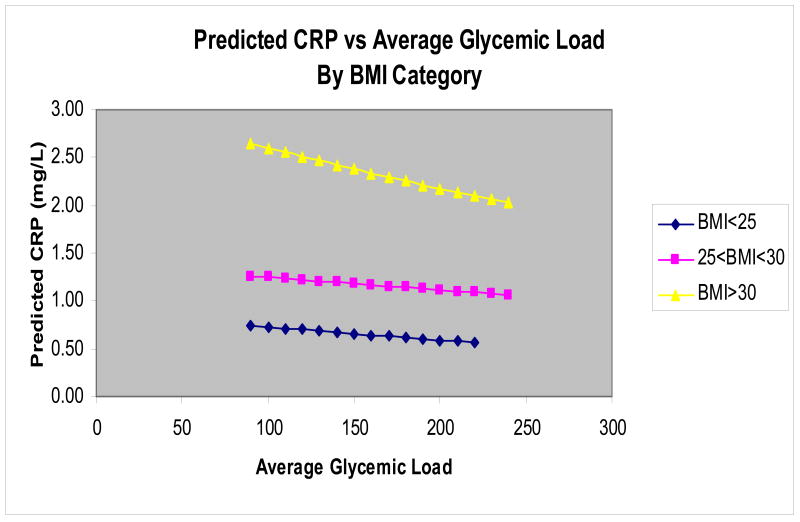
To further explore how BMI modified the relationship between dietary GL and hs-CRP, we fitted a linear model to clarify the interaction, without including any of the covariates. We then converted the results to the natural scale and plotted the predicted hs-CRP versus average GL (Figure 3). An inverse relationship was seen across all strata, with individuals in the highest BMI category having the greatest reduction of hs-CRP. However as GL increased, there was no statistical difference between the slope of obese participants and the slope of the other BMI categories (p values>0.05).
Figure 3. Predicted HS-CRP vs. Average Glycemic Load by BMI Category.
Discussion
In this one-year observational study, we did not observe a positive association between dietary GI, GL and hs-CRP. Although the literature reporting the relationship between GI or GL and hs-CRP is limited, the results from this study are not in agreement with a previous study that reported a significant positive association between dietary GL and hs-CRP [25].
One explanation for the difference in findings may be due to differences in the study populations. Among 244 middle-aged female participants in the Women's Health Study, Lui and colleagues reported that dietary GL was positively associated with hs-CRP [25]. In that study, the average GI and GL were 75 and 166, respectively, considered to be low GI and intermediate GL values compared to the intermediate GI of 85 and a high GL of 197 found in this study among both men and women. This suggests that, as a whole, the dietary carbohydrate quality of the current study participants was poorer and may limit our ability to detect an association between GI or GL and hs-CRP. In addition, average hs-CRP values were considerably lower in the present study as compared to the study by Liu [25]. Differences in age, BMI and smoking status may contribute to the differences observed between the two studies. However, both studies did controlled for age, BMI and smoking in the analysis, although residual confounding by these factors is still possible.
In a recent publication in examining the association of GI, GL, and cereal fiber intake with the risk of type 2 diabetes in a cohort of US black women, risk of diabetes was not statistically significantly associated with GL (26). The authors explained that it can be difficult to study GL because of its high correlation with total carbohydrate intake, because cereal fiber intake increased with quintiles of GL, while whole grains (a major source of cereal fiber) contributed to the GL. Previously dietary fiber has been associated lower CRP in this population (27).
There are a few limitations as well as strengths to this study. First, the cross-sectional study design does not allow for assessment of a temporal relationship because both carbohydrate quality and hs-CRP were measured at the same time points. However, by using multiple prospective measures in the longitudinal analysis we were able to determine how hs-CRP changes as GI or GL change, which provides an enhanced understanding of the relationship between dietary carbohydrate quality and hs-CRP.
Second, there may be potential confounders for which we were unable to control. It is possible that there are other unknown factors that influence hs-CRP and may be associated with GI and GL. The direction of the association would be unknown so the bias could be an over or underestimation of the true.
A strength of this study is the detailed dietary assessment. We used a comprehensive dietary assessment, collecting dietary information from up to fifteen 24 hour recalls. The recalls allowed us to collect detailed information on each individual food consumed. The GI of individual foods is very specific and can vary within food types, for example between types of breads having whole grain characteristics, differences in fiber, or with added influencing GI factors such as butter. Having such detailed information allowed for a more accurate calculation of GI. However, this study relied upon both published GI values and estimates derived from the 24hr recalls, and like all studies of GI a methodology to estimate the GI of untested foods.
Conclusion
In this prospective observational study, we found no association between dietary GI or GL and hs-CRP. This is a surprising finding, given a previous study's positive findings and the observation by our group that fiber, in agreement with other studies, was inversely associated with hs-CRP in our study population [27]. This is interesting because fiber is a very strong factor in the determination of GI, and one would expect that GI would also be associated. Due to the limited number of studies on this topic and the conflicting results further investigation is warranted.
Acknowledgments
This work was supported by grants: R01-HL52745, R21-HL074895-01, and R21-HL-076796-01 from the National Heart, Lung and Blood Institute (NHLBI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI. The authors thank Laura Robidoux, and Priscilla Cirillo for their assistance with study recruitment and data collection; Kelly Scribner for coordination of the 24-hour recalls; and SEASONS dietitians who conducted the 24-hour recalls: Susan Nelson, Christine Singelton, Pat Jeans, Karen Lafayette, Deborah Lamb, Stephanie Olson, and Eileen Capstraw. Dr Nader Rifai for his assistance with hs-CRP measurements; and Dr. Eric Rawson for his contribution in the development of the SEASONS hs-CRP project. We also thank Drs. Charles Matthews and Patty Freedson for their contribution on physical activity measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.www.cdc.gov/nchs/faststats/heart.htm Accessed April 15, 2007
- 2.www.cdc.gov/diabetes/pubs/estimates05.htm Accessed April 15, 2007
- 3.www.diabetes.org/diabetes-heart-disease-stroke.jsp Accessed April 15, 2007
- 4.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 5.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, Hutchinson WL. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 7.Rutter MK, Meigs JBL, Sullivan LM, D'Agostino RB, Wilson PWF. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110:380–385. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- 8.Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly Ds, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 9.Laaksonen DE, Niskanen L, Nyyssonen K, Punnonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia. 2004;47:1403–1410. doi: 10.1007/s00125-004-1472-x. [DOI] [PubMed] [Google Scholar]
- 10.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and the risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 11.Shuhei N, Kiminor Y, Nozomu K, Masamichi O, Nobuoki K. Elevated C-reactive protein is a risk factor for development of type 2 diabetes in Japanese Americans. Diabetes Care. 2003;26:2745–2757. doi: 10.2337/diacare.26.10.2754. [DOI] [PubMed] [Google Scholar]
- 12.Hu F, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Willett W, Stampher M, Hu F, Franz M, Sampson L, Hennekens CH. A prospective study of dietary glycemic load, carbohydrate intake and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 14.Schulze MB, Liu S, Rimm E, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80:348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 15.Brand-Miller J, Wolever TMS, Foster-Powell KF, Colagiuri S. The New Glucose Revolution. New York: Marlowe & Company; 2003. [Google Scholar]
- 16.Merriam PA, Ockene IS, Hebert Jr, Rosal MC, Matthews CE. Seasonal variation of blood cholesterol levels: Study Methodology. Journal of Biological Rhythms. 1999;14:330–339. doi: 10.1177/074873099129000669. [DOI] [PubMed] [Google Scholar]
- 17.http://www.nlm.nih.gov/medlineplus/ency/article/003356.htm Accessed May 12, 2007
- 18.Willet W. Nutritional Epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 19.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 20.http://www.glycemicindex.com/
- 21.Olendzki BC, Ma Y, Culver AL, Ockene IS, Griffith JA, Hafner AR, Hebert JR. Methodology for adding glycemic index and glycemic load values to 24-hour dietary recall database. Nutrition. 2006 Nov-Dec;22(1112):1087–95. doi: 10.1016/j.nut.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aronson D, Bartha P, Zinder O, Kerner A, Shitman E, Markiewicz W, Brook GJ. Association between fasting glucose and C-reactive protein in middle-aged subjects. Diabetic Medicine. 2003;21:39–44. doi: 10.1046/j.1464-5491.2003.01084.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller M, Zhan M, Havas S. High Attributable Risk of Elevated C-Reactive Protein Levels to Conventional Coronary Heart Disease Risk Factors. Arch Intern Med. 2005;165:2063–2068. doi: 10.1001/archinte.165.18.2063. [DOI] [PubMed] [Google Scholar]
- 24.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F. Markers of Inflammation and Cardiovascular Disease Applications to Clinical and Public Health Practice. A Statement for Healthcare Professionals From the Center for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Manson JE, Buring JE, Stampfer MJ, Willet WC, Ridker PM. Relation between a diet with a high dietary glycemic load and plasma concentrations of high sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75:492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan S, Rosenberg L, Singer M, Hu FB, Djousse L, Cupples LA, Palmer JR. Glycemic Index, Glycemic Load, and Cereal Fiber Intake and Risk of Type 2 Diabetes in US Black Women. Arch Intern Med. 2007;167:2304–9. doi: 10.1001/archinte.167.21.2304. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, Stanek EJ, 3rd, Li W, Pagoto SL, Hafner AR, Ockene IS. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006 Apr;83(4):760–766. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]