Abstract
Liver transplantation is currently the only curative treatment for patients with end-stage liver disease. However, the mechanisms underlying liver injury and hepatocyte proliferation post transplantation remain obscure. In this investigation, liver injury and hepatocyte proliferation in syngeneic and allogeneic animal models were compared. Male Lewis and Dark Agouti (DA) rats were subjected to orthotopic liver transplantation (OLT). Rat OLT was performed in syngeneic (Lewis-Lewis) and allogeneic (Lewis-DA or DA-Lewis) animal models. Allogeneic liver grafts exhibited greater injury and cellular apoptosis than syngeneic grafts, but less hepatocyte proliferation after OLT. Expression of IFN-γ mRNA and activation of the downstream signal (STAT1) and genes (IRF-1 and p21) were also greater in the allogeneic grafts compared with the syngeneic grafts. In contrast, STAT3 activation was lower in the allogeneic grafts. Furthermore, in the allogeneic grafts, depletion of NK cells decreased IFN-γ/STAT1 activation, but enhanced hepatocyte proliferation. These findings suggest that compared with syngeneic transplantation, innate immunity (NK/IFN-γ) is activated post allogeneic transplantation, which likely contributes to liver injury and inhibits hepatocyte proliferation.
Keywords: liver transplantation, liver regeneration, NK cells, STAT1, IFN-γ
Introduction
Chronic alcohol drinking, nonalcoholic steatohepatitis, viral hepatitis are the 3 major causes of chronic liver injury worldwide, causing end-stage liver diseases such as cirrhosis and hepatocellular carcinoma.(46) Currently, the only effective treatment for these end-stage diseases is liver transplantation. Of note, due to the liver's unique immunologic properties, the rejection rate for liver transplantation is lower compared with transplantation with other organs.(1, 39, 40) The liver's immune system is predominantly innate; enriched with innate immune cells such as Kupffer cells, natural killer (NK) cells, NKT cells, and γ/δ T cells.(9, 15, 36) In contrast, the adaptive immune response is less active in the liver where is the major site of induction of T cell apoptosis.(4, 5) Reportedly, within an acute allograft rejection model, NK cells are activated post transplantation, subsequently producing interferon-γ (IFN-γ) to trigger adaptive immune responses and ultimately acute rejection.(32) However, the effects of immune cells on liver injury and hepatocyte proliferation post transplantation remain obscure.
Liver lymphocytes are enriched in NK cells: approximately 5−10% in mice and 30−50% in rats and humans. (9, 15, 36) Not only do NK cells play a pivotal role in host defenses against pathogen invasion and tumor transformation, they are also involved in liver injury and repair.(11) In several different experimental models, NK cells have been shown to contribute to liver injury and inhibit liver fibrosis.(3, 10, 29, 33, 37) Studies have also shown that activated NK cells can directly kill hepatocytes or activated hepatic stellate cells via TRAIL- and NKG2D-dependent mechanisms.(3, 10, 29, 33, 37) In addition, NK cell activation inhibits liver regeneration by blocking hepatocyte and oval cell proliferation in an IFN-γ-dependent manner.(18, 41) NK cell activation produces IFN-γ, which targets hepatocytes by binding to IFN-γ receptors and activates the signal transducer and activator of transcription 1 (STAT1) signaling pathway. Activation of STAT1 induces cell cycle arrest and apoptosis in hepatocytes, thereby suppressing liver regeneration.(6, 42) In contrast to STAT1, activation of STAT3 appears to promote liver regeneration.(17, 27, 31, 47)
In this investigation, we examined the role of NK/IFN-γ on liver injury and hepatocyte proliferation in allogeneic and syngeneic orthotopic liver transplantation (OLT) models, where NK/IFN-γ is strongly activated in the allogeneic grafts, but not in the syngeneic grafts. There are two well-established models of allogeneic OLT: the spontaneous acceptance model of Lewis-to-DA rat liver transplants and the rejection model of DA-to-Lewis rat liver transplants. Livers transplanted from DA to Lewis rats are rejected within 9 to 12 days followed by resulting in death, but conversely, Lewis-to-DA liver transplants result in tolerance and survival.(12, 32) The mechanisms underlying tolerance of Lewis-to-DA liver transplants remain poorly understood. Previously, it was reported that in the Lewis-to-DA model, there is marked lymphocyte proliferation and infiltration, but it is unclear why this immune response does not lead to graft rejection.(2, 39) Our findings from this investigation reveal that although more liver injury was observed with the allogeneic (Lewis-to-DA) grafts compared with the syngeneic grafts, hepatocyte proliferation was lower in the allogeneic grafts. Compared with the syngeneic gratfts, higher levels of IFN-γ/STAT1 activation were detected in the allogeneic grafts. Depletion of NK cells decreased IFN-γ/STAT1 activation, but enhanced hepatocyte proliferation, suggesting that NK/IFN-γ is likely involved in decreased hepatocyte proliferation in the allogeneic grafts.
Materials and Methods
Animals
Inbred male Lewis and Dark Agouti (DA) rats weighing 220−260g were purchased from Harlan (Indianapolis, IN). All animals were kept in a temperature-controlled environment with a 12-hour light-dark cycle and were allowed free access to food and water at all times, and were cared for in accordance with National Institutes of Health (NIH) guidelines.
Surgical procedures
Rat orthotopic liver transplantation (OLT) was performed under anesthesia with isoflurane inhalation (in O2) as described previously.(43) Donor livers were flushed with 15 mL of cold saline solution (40 units/mL) and stored in 4°C until transplanted. The cold ischemia period lasted approximately 1 hour. Three types of transplantation groups were designed: group I syngeneic (Lewis-to-Lewis); group II allogeneic (Lewis-to-DA); group III allogeneic (DA-to-Lewis). Transplanted rats were killed post transplantation on days 1, 2, 3, 4, 5, and 7. In our lab, transplantation of Lewis-to-DA results in long-term survival, while transplantation of DA-to-Lewis results in acute organ rejection within 9 to 12 days, consistent with well-established reports.(12, 44)
Reverse transcription-polymerase chain reaction (RT-PCR)
Experiments were performed as described previously.(43) Rat primer sequences used in this study are listed in Table I.
Table I.
Rat primers for RT-PCR.
| Genes | Forward primers | Reverse primers | PCR size |
|---|---|---|---|
| IFN-γ | 5′-GCC CTC TCT GGC TGT TAC TG-3′ | 5′- CTG ATG GCC TGG TTG TCT TT-3′ | 221 bp |
| IL-6 | 5′-CCG GAG AGG AGA CTT CAC AG-3′ | 5′-ACA GTG CAT CAT CGC TGT TC-3′ | 161 bp |
| IL-2 | 5′-AAA CTC CCC ATG ATG CTC AC-3′ | 5′-GAA ATT TCC AGC GTC TTC CA-3′ | 156 bp |
| IL-15 | 5′-ATG TGA GGA GCT GGA GGA GA-3′ | 5′-TCA ACC GTT TCC TGT TAG GC-3′ | 205 bp |
| IRF-1 | 5′- GCT GCA ACA GAT GAG GAT GA-3′ | 5′- CTA TCT CGA TGT CCC CTC CA-3′ | 202 bp |
| p21 | 5′- TCA GTG GAC CAG AAG GGA AC-3′ | 5′- GGT CCC CAT CCC AGA TAA GT-3′ | 197 bp |
| SOCS1 | 5′-TGG TAG CAC GTA ACC AGG TG-3′ | 5′-GAA GGT GCG GAA GTG AGT GT-3′ | 176 bp |
| SOCS3 | 5′-CCT TTG AGG TTC AGG AGC AG-3′ | 5′-CGT TGA CAG TCT TCC GAC AA-3′ | 165 bp |
Immunohistochemistry
Paraffin-embedded sections were cut, deparaffinized, and hydrated by soaking in 100% xylene and descending ethanol, followed by heating treatment and incubation in 0.3% H2O2 in phosphase buffered saline (PBS) to block endogenous peroxidase activity. Slides were then stained with anti-PCNA or anti-Ki67 antibodies (1:50 dilution) (Zymed Laboratories, San Francisco, CA) overnight at 4°C in a moist chamber. Biotinylated secondary antibodies and ABC reagent were then applied. Color development was induced by incubation with the DAB kit (Vector Laboratories, Burlingame, CA) for 3 to 5 minutes, and specific staining was visualized by light microscopy.
Terminal uridine deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Staining was performed using an in situ apoptosis detection kit according to the manufacturer's instructions (Chemicon International, Temecula, CA) and examined by a light microscopy. The TUNEL positive hepatocytes were counted randomly in 10 fields (×200) of each slide. The percentage was calculated as number of TUNEL positive hepatocytes per total number of hepatocytes.
Western blotting
Liver tissues were homogenized in protein lysis buffer (30 mM Tris, PH 7.5, 150 mM sodium chloride, 1 mM sodium orthovanadate, 1% Nonidet P-40, 10% glycerol, and phosphatase and protease inhibitors). Western blot analyses were performed using 60 μg of protein from liver homogenates using STAT1, STAT3, p-STAT1, p-STAT3 antibodies (1:1000 dilution, Cell Signaling Technology, Danvers, MA).
Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST)
Serum levels of ALT and AST were measured using a kit from Drew Scientific Ltd (Barrow-in-Furness, UK).
Enzyme-linked immunosorbent assay (ELISA)
Serum levels of IFN-γ were measured using an ELISA kit (BioSource International, Camarillo, CA). All serum samples were analyzed in triplicate. This assay was determined to have a sensitivity of 10 pg/mL using recombinant rat IFN-γ as a standard (BioSource).
Depletion of NK cells and flow cytometric analysis
To deplete NK cells, donor and recipient rats were injected with anti-Rat NKRP1 antibody (100 μg/rat) (Endogen, Rockford, IL). After 24 hours, depletion of NK cells was confirmed by flow cytometric analysis by anti-rat CD3 and anti-rat NKRP1 antibodies (BD Biosciences, San Jose, CA).
Statistical analysis
Data are expressed as means ± SEM. To compare values obtained from two groups, the student's t-test was performed. To compare values obtained from three or more groups, one-way analysis of variance (ANOVA) was performed. A value of P<0.05 was considered significant.
Results
Liver injury and apoptosis are higher in the allogeneic grafts vs. the syngeneic grafts
Results show that serum ALT and AST levels in the allogeneic groups (L-DA, DA-L) on day 1 post surgery were slightly higher than the syngeneic group (L-L), but this difference did not reach statistical significance (Figs. 1 A-B). On the following days, serum ALT and AST levels in the syngeneic transplant group rapidly decreased. In contrast, ALT and AST values declined on days 2−3, but then started to increase on days 4, 5, and 7 in the allogeneic group. Increased serum levels of ALT and AST were more evident in the DA-L group than the L-DA group.
Figure 1.
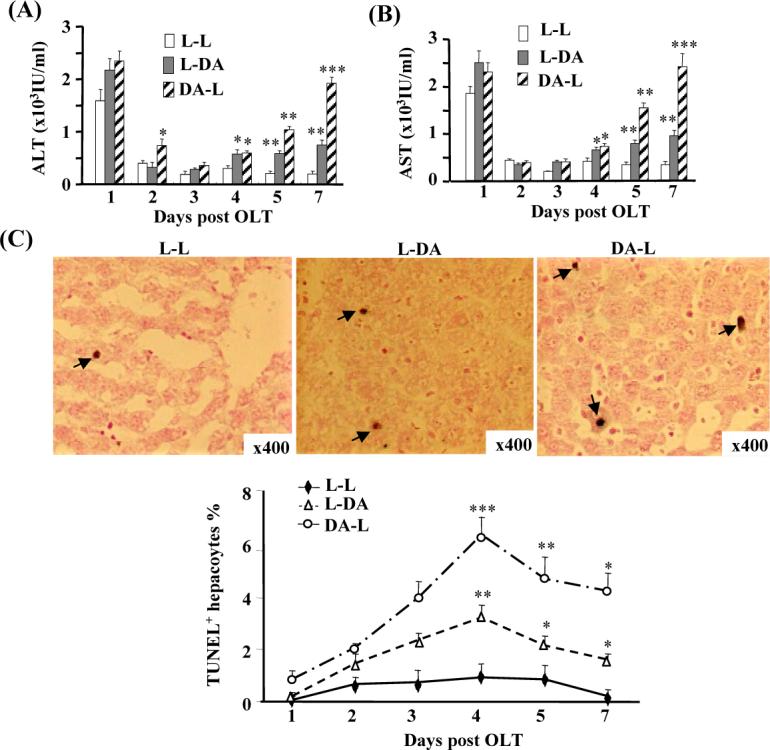
Liver injury and apoptosis are higher in the allogeneic grafts vs. the syngeneic grafts. Three pairs of OLT were performed (Lewis to Lewis: L-L; Lewis to DA: L-DA; DA to Lewis: DA-L) for various time points. (A, B) Sera were harvested for ALT/AST measurement. (C) Liver tissues were harvested and stained with TUNEL assay to examine apoptosis. Representative photomicrographs are shown in the top panel (arrows indicate TUNEL+ hepatocytes). The number of TUNEL+ hepatocytes was counted and shown in the bottom panel. Values in panels A-C are shown as means ± SEM (n=3 pairs on day 1; n=5 pairs on day 2 and 5; n=6 pairs on day 3, 4, and 7). *P<0.05, **P<0.01, ***P<0.001 compared with values from corresponding L-L syngeneic grafts.
In view of the increased liver injury found in the allogeneic grafts, we next examined whether the allografts had more apoptosis than the syngeneic grafts by TUNEL assay to detect the presence of apoptotic cells. As shown in Fig. 1C, peak hepatocyte apoptosis occurred 4 days post-transplant, with the most apoptosis observed in the DA-L group (6%), followed by the LDA group (3%), and the lowest in the L-L group (1%), which are consistent with previous reports.(25) Percentages of apoptotic TUNEL-positive hepatocytes were also higher in the L-DA and DA-L groups on day 3, 5, and 7 post-transplant compared with the L-L group. Similar to previous reports,(22, 30) we also observed significantly increased numbers of apoptotic lymphocytes in the allogeneic grafts. As this paper is mainly focused on hepatocyte proliferation and injury, the number of apoptotic lymphocytes was not counted in this paper.
Hepatocyte proliferation is lower in the allogeneic grafts vs. the syngeneic grafts
Hepatocyte proliferation was determined by immunostaining with Ki-67 or PCNA antibodies. Results shown in Fig. 2 reveal that peak Ki-67 staining occurred on day 3 after transplantation in all 3 groups, with the highest in the L-L group (approximately 12%), but only 2−5% in the L-DA and DA-L groups. Similarly, peak PCNA staining was also observed on day 3 post transplant in all 3 groups, with the most staining detected in the L-L group.
Figure 2.
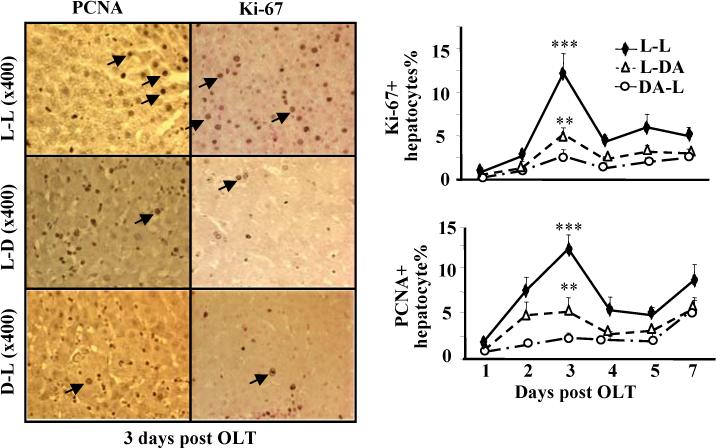
Hepatocyte proliferation is lower in the allogeneic grafts vs. the syngeneic grafts. The liver tissues from Fig. 1 were stained with anti-PCNA or anti-Ki67 antibodies. Representative photomicrographs are shown in left panel. The numbers of Ki67+ and PCNA+ hepatocytes were counted and shown in right panel. Values are shown as means ± SEM (n=3−6 as described in Fig. 1). **P<0.01, ***P<0.001 compared with values from corresponding L-L syngeneic grafts.
Upregulation of STAT1 activation and downregulation of STAT3 in the allogeneic grafts vs. the syngeneic grafts
The STAT3 protein has been implicated in promoting liver regeneration, while STAT1 has been implicated in inhibition of liver regeneration.(6, 17, 27, 31, 42, 47) To define the molecular mechanism by which liver regeneration is suppressed in allogeneic grafts, we examined STAT1 and STAT3 activation in all 3 groups. As shown in Figs. 3A-B, low levels of STAT1 activation was detected in syngeneic grafts, with peak activation on day 5. In contrast, stronger STAT1 activation was detected in the allogeneic grafts (L-DA or DA-L), with peak effect occurring 3 days after surgery. Accordingly, expression of STAT1 protein, which is known to be induced by activated STAT1,(24) was induced and expressed more in the allogeneic grafts (L-DA or DA-L) vs. the syngeneic grafts (L-L).
Figure 3.
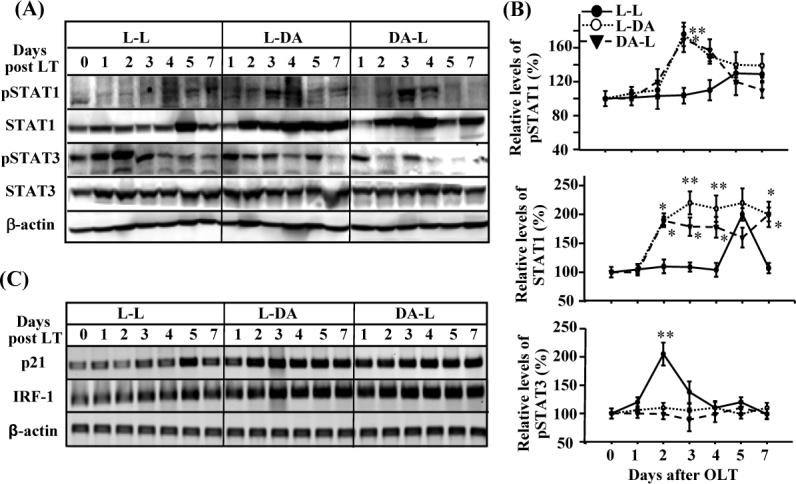
Upregulation of STAT1 activation and downregulation of STAT3 activation in the allogeneic grafts vs. the syngeneic grafts. The liver tissues from Fig. 1 were subject to Western blotting (A, B) and RT-PCR analyses (C). A representative of 3 experiments with similar results is shown. The band densities in panel A were quantified and the ratio of pSTAT1 (or STAT1, pSTAT3)/β actin mRNA were calculated. The ratios on day 0 were designated as 100%. Values are shown as means ± SEM (n=3−6 as described in Fig. 1). *P<0.05, **P<0.01 compared with values from corresponding L-L syngeneic grafts.
In contrast to high levels of STAT1 activation, STAT3 activation (p-STAT3) was lower in the allogeneic grafts (L-DA and DA-L) vs. the syngeneic grafts (L-L). As shown in Figs. 3A-B, peak expression of pSTAT3 was detected in the L-L group on day 2 post-transplant, consistent with previous reports.(7, 43) Expression of pSTAT3 was much lower in the L-DA or DA-L allogeneic grafts compared with the L-L syngeneic grafts.
Next, we also examined the expression of p21 and IRF-1, the genes downstream of STAT1, in the transplanted livers. As shown in Fig. 3C, expression of IRF-1 and p21 was significantly higher in the allogeneic grafts than the syngeneic grafts.
Upregulation of hepatic IFN-γ mRNA and serum IFN-γ levels in the allogeneic grafts vs. the syngeneic grafts
To understand the molecular mechanisms by which activation of STAT1 was enhanced while STAT3 was decreased in the allogeneic grafts, we examined the expression of several cytokines in the livers. As shown in Fig. 4A, expression of IFN-γ was much higher in the allogeneic grafts compared with the syngeneic grafts. In the L-L group, IFN-γ mRNA was only significantly induced 5 days after the surgery, while significant elevation of IFN-γ mRNA expression was observed 2 days after surgery and detected at high levels up to day 7 post-transplant in both LDA and DA-L groups. Hepatic IL-6 mRNA expression was also induced after OLT, with peak induction on days 2 and 3 post surgery in the syngeneic grafts, consistent with a previous report.(7) Induction of hepatic IL-6 mRNA was greater and prolonged in the L-DA and DA-L groups. Moreover, expression of IL-2 and IL-15 was slightly induced in all 3 groups, with slightly higher expression in the allogeneic grafts than the syngeneic grafts. Hepatic suppressor of cytokine signaling 1 (SOCS1) and SOCS3 mRNA expression were also significantly induced post-transplant in all groups. This expression was slightly higher in the allogeneic grafts vs. the synegenic grafts, which is likely due to higher levels of IFN-γ and IL-6 expression in the liver (Fig. 4A) since IFN-γ and IL-6 are responsible for induction of SOCS1 and SOCS3, respectively. (19)
Figure 4.
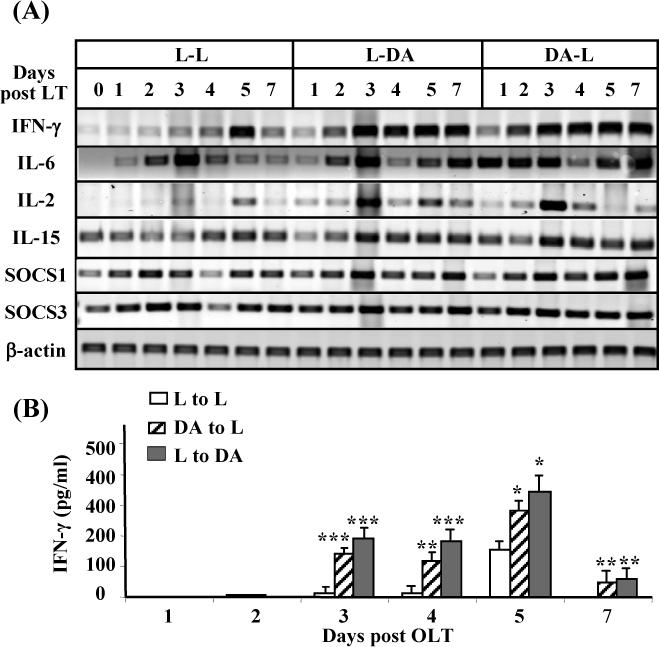
Upregulation of hepatic IFN-γ mRNA and serum IFN-γ levels in the allogeneic groups vs. the syngeneic groups. (A) The liver tissues from Fig. 1 were subject to RT-PCR analyses. A representative of 3 experiments with similar results is shown. (B) Sera from these transplanted rats were used for IFN-γ measurement. Values are shown as means ± SEM (n=3−6 as described in Fig. 1). *P<0.05, **P<0.01, ***P<0.001 compared with values from corresponding L-L syngeneic grafts.
Serum levels of IFN-γ were elevated 5 days post-OLT in the L-L group, which correlated with peak expression of hepatic IFN-γ mRNA (Fig. 4A) and peak activation of STAT1 (Fig. 3A) in this group. Interestingly, serum IFN-γ levels were elevated much earlier and higher in the allogeneic groups compared with the syngeneic groups (Fig. 4B), consistent with a previous report.(32) Higher serum IFN-γ levels in the allogeneic groups also correlated with higher levels of hepatic IFN-γ mRNA expression (Fig. 4A) and STAT1 activation (Fig. 3A).
Depletion of NK cells decreases STAT1 activation, but enhances liver regeneration in the allografts
It is known that NK cells are activated after OLT and are the major cells responsible for producing IFN-γ.(32) To define the role of NK cells in liver regeneration, the NKRP1 antibody was used to deplete NK cells in both donor and recipient rats. Injection of the NKRP1 antibody for 24 hours depleted completely liver NKRP1highCD3− NK cells and depleted 90% NKRP1mediumCD3+ NKT cells in both donor and recipient rats (only recipient DA rat flow cytometric data are shown in Fig. 5A). Immunohistochemistry analyses showed that the percentages of Ki-67 or PCNA labeled hepatocytes were higher in the allografts treated with NKRP1 antibodies compared to those treated with IgG (Figs. 5B-C). STAT1 activation and expression were lower in the NKRP1 antibody-treated groups than in the IgG-treated groups (Fig. 5D). Similarly, expression of IFN-γ, IRF-1, and p21 decreased in the NKRP1 antibody-treated groups compared with the IgG-treated groups (Fig. 5E).
Figure 5.
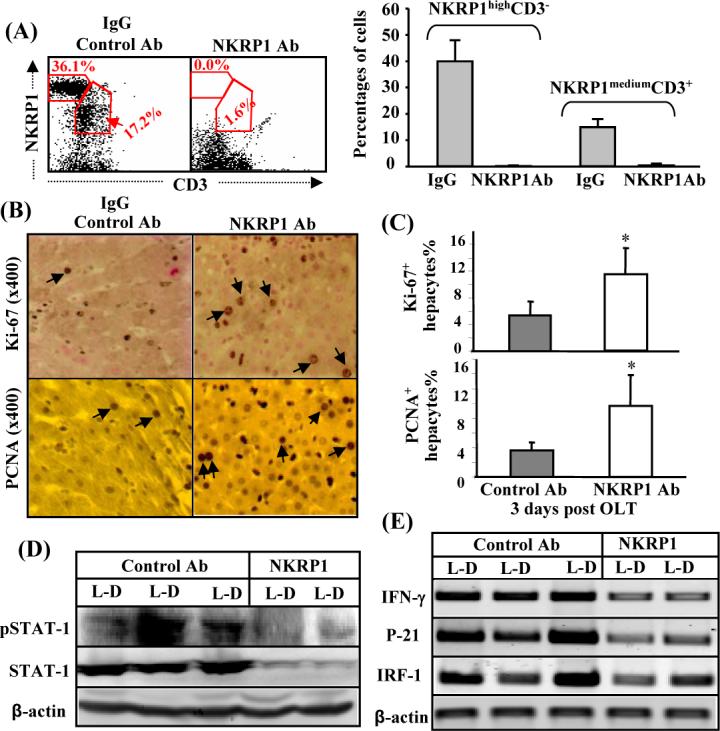
Depletion of NK cells enhances liver regeneration with deceasing STAT1 activation and IFN-γ/p21/IRF-1 mRNA expression in the allogeneic grafts. (A) Lewis and DA rats were treated with anti-NKR1P antibodies or IgG control antibodies. Twenty-four hours later, liver lymphocytes were isolated and analyzed with anti-CD3 and anti-NKRP1 antibodies. The representative flow cytometric results from DA rats are shown in the left panel. A summary of 3 independent experiments for the percentage of NK (NKRP1highCD3−) and NKT (NKRP1mediumCD3+) cells is shown in the right panel. (B-E) The livers from anti-NKR1P antibody- or control IgG-treated Lewis rats were transplanted into anti-NKRP1- or IgG-treated DA rats. Three days later, the transplanted DA rats were killed and livers harvested for immunostaining with anti-Ki67 or anti-PCNA antibodies. Representative photomicrographs are shown in panel B (arrows indicate Ki67+ or PCNA+ hepatocytes). The numbers of Ki67+ and PCNA+ hepatocytes were counted and are shown in C. Values are shown as means ± SEM (n=3−4). *P<0.05 compared with the corresponding IgG-treated allografts. Liver tissues were also subject to Western blotting (D) or RT-PCR analyses (E). A representative of 3 experiments with similar results is shown.
Discussion
In this investigation, we demonstrated that liver injury and apoptosis were higher but hepatocyte proliferation was lower in the allogeneic transplant rats compared with the syngenic transplant rats. Furthermore, we provided evidence suggesting (a) that upregulation of STAT1 activation and downregulation of STAT3 activation likely contribute to enhanced liver injury but suppressed hepatocyte proliferation in the allogeneic grafts compared with the syngenic grafts; (b) upregulation of IFN-γ production is responsible, at least in part, for stronger STAT1 activation in the allogeneic groups vs. the syngeneic group. NK cells likely contribute to IFN-γ production.
Downregulation of STAT3 and upregulation of STAT1 contribute to enhanced liver injury but decreased hepatocyte proliferation in allografts
Hepatic STAT3 is activated in all forms of liver injury, including liver transplantation.(7, 8, 14, 43) STAT3 activation likely contributes to hepatocyte proliferation, as ablation of the hepatic STAT3 gene diminished hepatocyte proliferation after partial hepatectomy or administration of CCl4.(17, 27, 31) Upregulation of IL-6 and STAT3 activation has been detected in liver transplants after prolonged cold ischemia, correlating with increased hepatocyte proliferation.(7) Moreover, overexpression of constitutively activated STAT3 via adenoviral gene transfer attenuates liver injury and promotes hepatocyte proliferation in a rat model of 20% partial liver transplantation.(20) In contrast to STAT3, STAT1 activation has been shown to inhibit hepatocyte proliferation induced by partial hepatectomy in mice and contribute to IFN-γ inhibition of mouse hepatocyte proliferation.(6, 42) The inhibitory effects of STAT1 in hepatocyte proliferation is mediated partly via induction of IRF-1 and p21 gene expression, followed by induction of cell cycle arrest and apoptosis in hepatocytes.(42) Interestingly, compared with the syngeneic grafts, STAT3 activation was significantly lower while STAT1 was higher in the allogeneic grafts (L-DA or DA-L) (Fig. 3). Accordingly, the STAT1 downstream genes, IRF-1 and p21, were also upregulated in the allogeneic grafts (Fig. 3). Taken together, these findings strongly suggest that upregulation of STAT1 and downregulation of STAT3 are likely involved in decreasing hepatocyte proliferation and increasing apoptosis in the allogeneic grafts. Although STAT1 may contribute to decreased hepatocyte proliferation and increased apoptosis in the allogeneic grafts, STAT1 does not contribute to or correlate with the survival rate because STAT1 protein expression was significantly induced in both L-DA (100% survival rate) and DA-L groups (0% survival rate).
Upregulation of IFN-γ likely contributes to increased STAT1 activation in the synegenic transplant groups
IFN-γ is one of the major cytokines that activate STAT1 in the liver.(14) Here we demonstrated that serum levels of IFN-γ protein and hepatic IFN-γ mRNA are much greater in the allogeneic groups vs. the syngeneic group (Fig. 4), and correlate with STAT1 activation and apoptosis (TUNEL) in the allogeneic grafts. This strongly suggests that higher levels of IFN-γ are responsible for inducing stronger STAT1 activation and apoptosis in the allogeneic group. Interestingly, the peak level of IFN-γ did not correlate with the peak level of ALT on day 1 post transplantation in Fig. 1. This is probably because peak levels of ALT on day 1 were caused by ischemia/reperfusion liver injury and IFN-γ was not involved. IFN-γ and activation of STAT1 likely contribute to the apoptosis and elevation of ALT/AST on day 3−7 post transplantation. Consistent with our findings, higher serum levels of IFN-γ protein and hepatic IFN-γ mRNA in the allogeneic transplantation than in the syngeneic group were also previously reported.(21, 32) Interestingly, Lord et al (28) reported that hepatic IFN-γ mRNA expression was higher in a model of allogeneic transplantation (DA-to-PVG) than in syngeneic transplantation (DA-to-DA), but hepatic IFN-γ protein expression was similar in both groups after transplantation.(28) The reason for higher levels of hepatic IFN-γ mRNA, but not hepatic IFN-γ protein, in the syngeneic group may be due to the rapid secretion of hepatic IFN-γ protein into the blood stream.
The next obvious question for our laboratory was what kinds of cells are responsible for IFN-γ production in the allogeneic transplantation groups. The findings that treatment with anti-NKRP1 antibodies reduced hepatic IFN-γ mRNA expression (Fig. 5) and treatment with anti-ASGM1 antibodies reduced serum IFN-γ levels in allogeneic transplanted rats (32) suggest that both NK (NKRP1highCD3−) cells and NKT (NKRP1mediumCD3+) cells may be responsible for IFN-γ production since treatment with anti-NKRP1 or anti-ASGM1 antibodies depleted both types of cells. Furthermore, the flow cytometry analyses reported by Obara et al(32) showed that the NK (NKRP1highCD3−) cells and activated T cells contribute to IFN-γ production in the allogeneic groups, while NKT (NKRP1mediumCD3+) cells seem not involved (reference (32) and Dr. Sheri Krams' personal communication). At present, it is not clear why infiltrating NK cells in the allogeneic transplant groups produced much higher IFN-γ levels compared with those in the syngeneic transplant groups. It is plausible to speculate that in the syngeneic groups, infiltrating NK cells encounter syngeneic cells and the killing of syngeneic cells is prevented as NK cells co-express clonally distributed receptors for self-MHC class I molecules. On the other hand, in the allogenic groups, infiltrating NK cells interact with mismatched allogeneic targets and sense the missing expression of self-MHC class I molecules and subsequently become alloreactive (‘missing self’ theory).(26) Moreover, depletion of NK cells reduced expression of IFN-γ and STAT1 activation in the liver, correlating with enhanced liver regeneration after NK cell depletion (Fig. 5). These findings clearly suggest that activation of NK cells inhibits liver regeneration via production of IFN-γ and subsequent activation of STAT1 in the allogeneic transplant groups.
In contrast to STAT1, activation of STAT3 was suppressed in the allogeneic groups compared with the synegeneic group. The finding that hepatic IL-6, a major cytokine responsible for activating STAT3 in the liver,(14) were higher in the allogeneic groups compared with the synegeneic groups suggest that lower STAT3 activation was not due to lack of IL-6 expression. Expression of SOCS1 and SOCS3, the two major inhibitor proteins for STAT3, was similar in the allogeneic and syngeneic groups, indicating that decreased STAT3 activation in the former is not due to upregulation of SOCS1/3. In addition to SOCSs, STAT3 signaling can be suppressed by other factors such as PIAS3, protein tyrosine phsophatase, MAP kinase, PKC etc.(16) It is of interest to determine whether these inhibitory pathways for STAT3 are increased in the allogeneic group compared with the syngeneic groups.
Finally, it has been reported in a variety of models that liver disease progression is controlled tightly by the mutual antagonism of IFN-γ/STAT1 and IL-6/STAT3, which negatively regulates one another.(19, 23, 34, 38) Such mutual antagonism and regulation are likely involved in the allogeneic transplantation model. For example, increased STAT1 signaling may contribute to decreased STAT3 signaling in the allogeneic grafts. Modulation of the balance between STAT1 and STAT3 could offer a novel approach in protecting against liver injury and promoting liver regeneration in allogeneic liver transplantation.
Implications of this study
Increased serum levels of IFN-γ have also been detected in human liver transplant patients with organ rejection, but not in patients with organ acceptance,(13, 35, 45) suggesting that IFN-γ may be involved in contribution of liver injury but suppression of hepatocyte proliferation in some human allograft livers. The reason why serum and hepatic IFN-γ levels were not higher in the majority of patients with allograft liver transplantation is probably due to the application of immunosuppressive drugs, as treatment with these drugs have shown to block hepatic IFN-γ mRNA expression.(21) More attention should be paid to hepatocyte proliferation in liver transplant patients with elevated serum IFN-γ levels, which could be a therapeutic target to improve hepatic repair in these patients.
Abbreviations
- OLT
orthotopic liver transplantation
- DA rats
Dark Agouti rats
- NK cells
natural killer cells
- IFN-γ
interferon-γ
- STAT
signal transducer and activator of transcription factor
- pSTAT
phosphorylated STAT
- SOCS
suppressor of cytokine signaling
- IRF-1
Interferon regulatory factor 1
- p21
cyclin-dependent kinase inhibitor p21CDKN1A.
References
- 1.Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007;27:194–213. doi: 10.1055/s-2007-979471. [DOI] [PubMed] [Google Scholar]
- 2.Bishop GA, Wang C, Sharland AF, McCaughan G. Spontaneous acceptance of liver transplants in rodents: evidence that liver leucocytes induce recipient T-cell death by neglect. Immunol Cell Biol. 2002;80:93–100. doi: 10.1046/j.1440-1711.2002.01049.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Wei H, Sun R, Dong Z, Zhang J, Tian Z. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology. 2007;46:706–715. doi: 10.1002/hep.21872. [DOI] [PubMed] [Google Scholar]
- 4.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 5.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 6.Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, Cam MC, Gao B, Robinson GW, Hennighausen L. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46:504–513. doi: 10.1002/hep.21713. [DOI] [PubMed] [Google Scholar]
- 7.Debonera F, Aldeguer X, Shen X, Gelman AE, Gao F, Que X, Greenbaum LE, Furth EE, Taub R, Olthoff KM. Activation of interleukin-6/STAT3 and liver regeneration following transplantation. J Surg Res. 2001;96:289–295. doi: 10.1006/jsre.2001.6086. [DOI] [PubMed] [Google Scholar]
- 8.Debonera F, Wang G, Xie J, Que X, Gelman A, Leclair C, Xin D, Shaked A, Olthoff KM. Severe preservation injury induces Il-6/STAT3 activation with lack of cell cycle progression after partial liver graft transplantation. Am J Transplant. 2004;4:1964–1971. doi: 10.1111/j.1600-6143.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 9.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 10.Dong Z, Wei H, Sun R, Hu Z, Gao B, Tian Z. Involvement of natural killer cells in PolyI:C-induced liver injury. J Hepatol. 2004;41:966–973. doi: 10.1016/j.jhep.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Dong ZJ, Wei HM, Sun R, Tian ZG. The roles of innate immune cells in liver injury and regeneration. Cell Mol Immunol. 2007;4:241–252. [PubMed] [Google Scholar]
- 12.Dresske B, Lin X, Huang DS, Zhou X, Fandrich F. Spontaneous tolerance: experience with the rat liver transplant model. Hum Immunol. 2002;63:853–861. doi: 10.1016/s0198-8859(02)00448-2. [DOI] [PubMed] [Google Scholar]
- 13.Ganschow R, Broering DC, Nolkemper D, Albani J, Kemper MJ, Rogiers X, Burdelski M. Th2 cytokine profile in infants predisposes to improved graft acceptance after liver transplantation. Transplantation. 2001;72:929–934. doi: 10.1097/00007890-200109150-00031. [DOI] [PubMed] [Google Scholar]
- 14.Gao B. Cytokines, STATs and liver disease. Cell Mol Immunol. 2005;2:92–100. [PubMed] [Google Scholar]
- 15.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 16.Greenhalgh CJ, Hilton DJ. Negative regulation of cytokine signaling. J Leukoc Biol. 2001;70:348–356. [PubMed] [Google Scholar]
- 17.Haga S, Ogawa W, Inoue H, Terui K, Ogino T, Igarashi R, Takeda K, Akira S, Enosawa S, Furukawa H, Todo S, Ozaki M. Compensatory recovery of liver mass by Akt-mediated hepatocellular hypertrophy in liver-specific STAT3-deficient mice. J Hepatol. 2005;43:799–807. doi: 10.1016/j.jhep.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Hines IN, Kremer M, Isayama F, Perry AW, Milton RJ, Black AL, Byrd CL, Wheeler MD. Impaired liver regeneration and increased oval cell numbers following T cell-mediated hepatitis. Hepatology. 2007;46:229–241. doi: 10.1002/hep.21674. [DOI] [PubMed] [Google Scholar]
- 19.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, Nguyen VA, Gao B. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–1513. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huda KA, Guo L, Haga S, Murata H, Ogino T, Fukai M, Yagi T, Iwagaki H, Tanaka N, Ozaki M. Ex vivo adenoviral gene transfer of constitutively activated STAT3 reduces post-transplant liver injury and promotes regeneration in a 20% rat partial liver transplant model. Transpl Int. 2006;19:415–423. doi: 10.1111/j.1432-2277.2006.00285.x. [DOI] [PubMed] [Google Scholar]
- 21.Jia C, Zheng S, Xie H. CsA downregulates IFN-gamma gene transcription after liver transplantation by inhibiting NF-kappa B activity. Chin Med J (Engl) 2003;116:1668–1672. [PubMed] [Google Scholar]
- 22.Jonsson JR, Gu W, Vanags DM, Bishop GA, McCaughan GW, Fawcett J, Lynch SV, Balderson GA, Powell EE, Clouston AD. Increased mononuclear cell activation and apoptosis early after human liver transplantation is associated with a reduced frequency of acute rejection. Liver Transpl. 2004;10:397–403. doi: 10.1002/lt.20084. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura H, Govindarajan S, Aswad F, Machida K, Lai MM, Sung VM, Dennert G. HCV core expression in hepatocytes protects against autoimmune liver injury and promotes liver regeneration in mice. Hepatology. 2006;44:936–944. doi: 10.1002/hep.21360. [DOI] [PubMed] [Google Scholar]
- 24.Kim WH, Hong F, Radaeva S, Jaruga B, Fan S, Gao B. STAT1 plays an essential role in LPS/D-galactosamine-induced liver apoptosis and injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G761–768. doi: 10.1152/ajpgi.00224.2003. [DOI] [PubMed] [Google Scholar]
- 25.Krams SM, Egawa H, Quinn MB, Villanueva JC, Garcia-Kennedy R, Martinez OM. Apoptosis as a mechanism of cell death in liver allograft rejection. Transplantation. 1995;59:621–625. [PubMed] [Google Scholar]
- 26.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277:28411–28417. doi: 10.1074/jbc.M202807200. [DOI] [PubMed] [Google Scholar]
- 28.Lord R, Goto S, Pan T, Chiang K, Chen C, Sunagawa M. Peak protein expression of IL-2 and IFN-gamma correlate with the peak rejection episode in a spontaneously tolerant model of rat liver transplantation. Cytokine. 2001;13:155–161. doi: 10.1006/cyto.2000.0815. [DOI] [PubMed] [Google Scholar]
- 29.Melhem A, Muhanna N, Bishara A, Alvarez CE, Ilan Y, Bishara T, Horani A, Nassar M, Friedman SL, Safadi R. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Meyer D, Thorwarth W, Otto C, Gasser M, Gassel H, Timmermann W, Ulrichs K, Thiede A. Early T-cell inactivation and apoptosis-critical events for tolerance induction after allogeneic liver transplantation. Transplant Proc. 2001;33:256–258. doi: 10.1016/s0041-1345(00)02003-0. [DOI] [PubMed] [Google Scholar]
- 31.Moh A, Iwamoto Y, Chai GX, Zhang SS, Kano A, Yang DD, Zhang W, Wang J, Jacoby JJ, Gao B, Flavell RA, Fu XY. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest. 2007;87:1018–1028. doi: 10.1038/labinvest.3700630. [DOI] [PubMed] [Google Scholar]
- 32.Obara H, Nagasaki K, Hsieh CL, Ogura Y, Esquivel CO, Martinez OM, Krams SM. IFN-gamma, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. Am J Transplant. 2005;5:2094–2103. doi: 10.1111/j.1600-6143.2005.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochi M, Ohdan H, Mitsuta H, Onoe T, Tokita D, Hara H, Ishiyama K, Zhou W, Tanaka Y, Asahara T. Liver NK cells expressing TRAIL are toxic against self hepatocytes in mice. Hepatology. 2004;39:1321–1331. doi: 10.1002/hep.20204. [DOI] [PubMed] [Google Scholar]
- 34.Ogata H, Kobayashi T, Chinen T, Takaki H, Sanada T, Minoda Y, Koga K, Takaesu G, Maehara Y, Iida M, Yoshimura A. Deletion of the SOCS3 gene in liver parenchymal cells promotes hepatitis-induced hepatocarcinogenesis. Gastroenterology. 2006;131:179–193. doi: 10.1053/j.gastro.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 35.Platz KP, Mueller AR, Rossaint R, Steinmuller T, Lemmens HP, Lobeck H, Neuhaus P. Cytokine pattern during rejection and infection after liver transplantation--improvements in postoperative monitoring? Transplantation. 1996;62:1441–1450. doi: 10.1097/00007890-199611270-00011. [DOI] [PubMed] [Google Scholar]
- 36.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 37.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 38.Siebler J, Wirtz S, Weigmann B, Atreya I, Schmitt E, Kreft A, Galle PR, Neurath MF. IL-28A is a key regulator of T-cell-mediated liver injury via the T-box transcription factor T-bet. Gastroenterology. 2007;132:358–371. doi: 10.1053/j.gastro.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 39.Starzl TE, Lakkis FG. The unfinished legacy of liver transplantation: emphasis on immunology. Hepatology. 2006;43:S151–163. doi: 10.1002/hep.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumpter TL, Abe M, Tokita D, Thomson AW. Dendritic cells, the liver, and transplantation. Hepatology. 2007;46:2021–2031. doi: 10.1002/hep.21974. [DOI] [PubMed] [Google Scholar]
- 41.Sun R, Gao B. Negative regulation of liver regeneration by innate immunity (natural killer cells/interferon-gamma). Gastroenterology. 2004;127:1525–1539. doi: 10.1053/j.gastro.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 42.Sun R, Park O, Horiguchi N, Kulkarni S, Jeong WI, Sun HY, Radaeva S, Gao B. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology. 2006;44:955–966. doi: 10.1002/hep.21344. [DOI] [PubMed] [Google Scholar]
- 43.Sun Z, Klein AS, Radaeva S, Hong F, El-Assal O, Pan HN, Jaruga B, Batkai S, Hoshino S, Tian Z, Kunos G, Diehl AM, Gao B. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology. 2003;125:202–215. doi: 10.1016/s0016-5085(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 44.Sun Z, Wada T, Maemura K, Uchikura K, Hoshino S, Diehl AM, Klein AS. Hepatic allograft-derived Kupffer cells regulate T cell response in rats. Liver Transpl. 2003;9:489–497. doi: 10.1053/jlts.2003.50091. [DOI] [PubMed] [Google Scholar]
- 45.Wang YL, Tang ZQ, Gao W, Jiang Y, Zhang XH, Peng L. Influence of Th1, Th2, and Th3 cytokines during the early phase after liver transplantation. Transplant Proc. 2003;35:3024–3025. doi: 10.1016/j.transproceed.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 47.Yeoh GC, Ernst M, Rose-John S, Akhurst B, Payne C, Long S, Alexander W, Croker B, Grail D, Matthews VB. Opposing roles of gp130-mediated STAT-3 and ERK-1/ 2 signaling in liver progenitor cell migration and proliferation. Hepatology. 2007;45:486–494. doi: 10.1002/hep.21535. [DOI] [PubMed] [Google Scholar]


