Abstract
Histophilus somni is a gram negative coccobacillus that causes respiratory and reproductive disease in cattle. The hallmark of systemic H. somni infection is diffuse vascular inflammation that can lead to an acute central nervous system disease known as thrombotic meningoencephalitis (TME). Previously we demonstrated that H. somni and its LOS activate bovine platelets leading to expression of P-selectin, CD40L and FasL. Since activated platelets have been reported to induce endothelial cell cytokine production and adhesion molecule expression we sought to determine if bovine platelets induce pro-inflammatory and pro-coagulative changes in bovine pulmonary artery endothelial cells. Endothelial cells were incubated with platelets activated with ADP, H. somni or H. somni LOS. Incubation with activated bovine platelets significantly increased expression of adhesion molecules (ICAM-1, E-selectin) and tissue factor, as measured by flow cytometry, real-time PCR, and western blot analysis. Activated platelets also up-regulated expression of endothelial cell IL-1β, MCP-1, and MIP-1α as determined by real-time PCR and an IL-1β ELISA. An interesting and surprising finding was that bovine platelets activated by H. somni or its LOS were internalized by bovine endothelial cells as visualized by transmission electron microscopy. This internalization appeared to correlate with endothelial cell activation and morphological changes indicative of cell stress. These findings suggest that activated platelets might play a role in promoting vascular inflammation during H. somni infection.
Keywords: Haemophilus, cytokine, E-selectin, tissue factor, ICAM-1, bovine
Introduction
Histophilus somni is a gram negative bacterial pathogen of cattle that causes respiratory and reproductive disease. A member of the Pastuerallaceae family, H. somni is one of the bacterial agents responsible for the bovine respiratory disease (BRD) complex, commonly known as “shipping fever” 1, 2. Infection with H. somni can lead to pneumonia, abortion, vascular inflammation, myocarditis, arthritis, multiple thrombotic lesions, and an acute form of vasculitis known as thrombotic meningoencephalitis (TME) 3–7. The molecular pathogenesis of H. somni in poorly understood. Known virulence factors of H. somni include resistance to serum-mediated killing, immunoglobulin binding proteins, and resistance to killing by phagocytes 4, 6, 8–11. H. somni produces a truncated form of lipopolysaccharide known as lipooligosaccharide (LOS), which is capable of undergoing phase variation 12. H. somni has also been reported to produce histamine and incorporate phosphorylcholine into its LOS, which may also influence its pathogenesis 13, 14.
How H. somni promotes vascular damage is poorly understood. Antigen-antibody complexes are not likely responsible, as colostrum-deprived calves without anti-H. somni antibodies can develop vasculitis 5. Another possibility is direct interactions between H. somni and the endothelium15. Previously, our lab has reported that H. somni and its LOS can induce caspase activation and apoptosis in bovine endothelial cells 16, 17. Although H. somni is commonly found associated with lesions in the lung and sometimes in thrombi, H. somni is not found in many vascular lesions 5. Because of this, we have investigated the participation of platelets in H. somni induced vasculitis. Besides their primary role in maintaining haemostasis, platelets also contribute to vascular inflammation and injury. It has been previously reported that activated human platelets can induce endothelial cell up-regulation of adhesion molecules (P-selectin, E-selectin, ICAM-1), chemokine release (MCP-1, MIP-1α, IL-8), IL-1β and tissue factor expression in co-culture systems 18–20.
We have reported previously that bovine platelets activated by H. somni or its LOS express pro-inflammatory molecules such as CD40L, P-selectin and FasL 21. In addition, activated bovine platelets could also induce endothelial cell apoptosis in a co-culture model. In this study, we demonstrate that bovine platelets activated by H. somni and its LOS, can promote endothelial cell pro-inflammatory responses. Following incubation with activated platelets, bovine endothelial cells expressed increased levels of adhesion molecules (ICAM-1, E-selectin), chemokines and IL-1β. Incubation with activated platelets also increased endothelial cell expression of tissue factor, which may promote coagulation and thrombus formation in vivo. We also observed that platelets activated by H. somni or its LOS demonstrated increased adherence to endothelial cells and significant numbers of platelets were also internalized. These data suggest that bovine platelets might play a role in promoting vascular inflammation and coagulation following activation by H. somni and its LOS.
Materials and Methods
Chemicals and Media
Adenosine diphosphate (ADP), Paraformaldehyde, thiamine monophophate, penicillin, streptomycin, and Dulbecco’s modified Eagle’s medium (DMEM, containing phenol red, 25 mM HEPES, 4.5 g/L dextrose and 2 mM L-glutamine) were obtained from Sigma Chemical Co., (St. Louis, MO). Brain heart infusion broth (BHI) and yeast extract were obtained from Difco (Detroit, MI). Mouse monoclonal antibodies against ICAM-1 were purchased from Zymed (Carlsbad, California). FITC-conjugated anti-rabbit antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Antibodies against von Willibrand Factor were purchased from Chemicon (Temecula, CA). Antibodies against CD146 were purchased from Serotec (Raleigh, NC). Antibodies against tissue factor were purchased from Enzyme Research (South Bend, IN). Karnovsky’s fixative and other electron microscopy supplies were purchased from Electron Microscopy Sciences (Hatfield, PA).
Platelet preparation
Healthy donor cattle were housed and bled following an approved animal care committee protocol. Cattle were bled by coccygeal venipuncture using an 18 G needle and vacutainer tubes containing sodium citrate (0.38% v/v) as anticoagulant. To minimize platelet activation during blood collection, care was taken to obtain venipuncture at the first needle stick. Blood from the first collection tube was discarded because this tube was more likely to contain platelets activated by the process of venipuncture. The collected blood was transferred to 50 ml polypropylene conical tubes and centrifuged at 500 × g for 10 minutes. The platelet rich plasma (PRP) was collected and the platelets enumerated with a hemocytometer after a 1:100 dilution in Tyrodes/HEPES buffer (138 mM NaCl, 2.9 mM KCl, 1 mM MgCl2, 1 mM glucose, 0.5 mM NaH2PO4, 20 mM HEPES, 0.3% BSA, pH 7.4). For purposes of these experiments, PRP was diluted in Tyrodes/HEPES buffer to a concentration of 1 × 107–108 platelets/ml. The platelets did not appear to be activated by the process of blood collection or processing as determined by flow cytometry evaluation of surface markers. Leukocyte contamination of the platelet preparations, as determined by Diff-Quik staining (VWR; West Chester, PA) was less than 104 leukocytes per ml.
Cultivation of bacterial strains
The pathogenic H. somni strain 649 was originally obtained from an aborted fetus and has been shown to cause reproductive failure when administered intrabronchially or intravenously. H. somni was incubated for 16 hours at 37°C, without shaking, in 25 cm2 tissue culture flasks containing 10 ml of BHI broth supplemented with 0.5% yeast extract and 0.05% thiamine monophosphate (BHI-YT). The number of colony forming units (CFU) per ml was determined by measuring absorbance (OD600), and extrapolating from a standard curve of H. somni grown under the same conditions. Frozen stock cultures of each H. somni isolate were stored at −70°C in BHI-YT and 10% glycerol. A fresh aliquot was thawed for use in each experiment. Lipooligosaccharide (LOS) was isolated by enzymatic digestion and hot phenol extraction from pathogenic strain 649 of H. somni as described previously 22.
Treatment of platelets
Approximately 1 × 10 7–108 platelets were added to 1.5 ml microcentrifuge tubes with Tyrodes/HEPES buffer (final volume 1 ml). Platelets were incubated with either H. somni (MOI 10:1) or H. somni LOS (500 ng/ml). Controls included untreated platelets and platelets stimulated with ADP (10 μM), as a positive control. Microcentrifuge tubes containing the platelet reactions were placed on a Labquake rotator (Barnstead International Dubuque, IA) and incubated at 37°C for 10 minutes. After incubation, 200 μl of 4% paraformaldehyde in PBS was added to each tube (final concentration 0.66% v/v) and the platelets were fixed for 30 minutes at 4°C. Following fixation, platelets were washed 3× in phosphate buffered saline. These conditions did not activate platelets as evaluated by expression of surface markers by flow cytometry.
Bovine pulmonary endothelial cells
The primary cultures of bovine endothelial cells used in this study were obtained by gentle scraping of the pulmonary artery collected from healthy steers at the time of slaughter, as described previously 16. These endothelial cells were cultured in DMEM (Cellgro, Herndon, VA) containing penicillin and streptomycin (500 IU/ml, Cellgro) and 20% fetal bovine serum (Atlanta Biologicals, Norcross, GA). The endothelial cells were seeded at a density of 1 × 105 cells/ml in 6 well tissue culture plates, and allowed to adhere overnight.
Treatment of endothelial cells
Washed platelets were incubated with endothelial cells at a density of approximately 10:1. To encourage a close association between platelets and endothelial cells the tissue culture plates were centrifuged at 500 ×g for 10 minutes. Endothelial cells were then incubated at 37°C for 1–6 hours.
Real-time RT-PCR
Quantitative real time PCR was used to measure the changes in cytokine mRNA levels in endothelial cells following incubation with bovine platelets. The Qiagen RNeasy minikit system (Qiagen, Valencia, CA) was used to collect mRNA from endothelial cells, following the manufacturers recommended protocol. The mRNA was converted to cDNA using the AMV Reverse Transcription System (Promega, Madison, WI) and cDNA samples were kept at −20 °C until needed. Primers used to amplify IL-1β, IL-8, MCP-1, MIP-1α, E-selectin, ICAM-1, tissue factor and the housekeeping gene β-actin were designed using the Primer Express software program (Primer Express 2.0; Applied Biosystems, Foster City, CA) using bovine sequences for these genes (IL-1β, GenBank accession M35589; MCP-1, GenBank accession M84602; MIP-1α, GenBank accession AY077840; E-selectin, GenBank accession L12039; tissue factor, GenBank accession NM_173878 and β-actin, GenBank accession AY141970). Primers used in this study are listed below:
5′ 3′
IL-1β forward: ATTGCCCAGGTTTCTGAAACA
IL-1β reverse: CTCGTCACTGTAGTAAGCCATCATTT
MCP-1α forward: TAATCCTCTCGCTGCAACA
MCP-1α reverse: TGCTGAAGGCAGCTACTGTGA
MIP-1 forward: GGTGTCATCTTCCAGACCAAAAA
MIP-1 reverse: TCC TGG ACC CAG TCC TCA GT
E-selectin forward: ACAAGCAGCCAACATGTAAAGCT
E-selectin reverse: AACCACGGAGTGGCTACAGTTC
Tissue factor forward: CGG TAC AAG ATG CAC GTA CGT
Tissue factor reverse: AAT TCA AGT CCT TGC CAA AAA CA
β-actin forward: AACCGTGAGAAGATGACCCAGAT
β-actin reverse: GGGACAGCACAGCCTGGAT
All primers were designed to amplify approximately 100 bp of the desired gene, have a melting point of 60 °C, and to minimize formation of primer-dimers. A nucleotide–nucleotide basic local alignment search tool (US National Library of Medicine, Bethesda, MD) was performed on the amplified regions to confirm the uniqueness of the PCR product. The forward and reverse primers (100 nM each) were combined with SYBR Green PCR Master Mix (Applied Biosystems), and cDNA template in a 96 well optical reaction plate (Applied Biosystems). The samples were cycled on a GeneAmp 7300 Real Time PCR System (Applied Biosystems). The cycle parameters were as follows: 50 °C for 2 min, 95 °C for 15 minutes, and for 40 cycles, 95 °C for 15 seconds and 60 °C for 1 minute. A cut-off of 1 was chosen for the threshold value for the amplicon levels. Fold increase in mRNA was calculated by the comparative threshold cycle (CT) method using the formula 2ΔΔCT. Data were normalized for gene expression based on β-actin mRNA expression.
Western blot analysis
Endothelial cells (1 × 105/ml) in 6 well plates were washed in PBS, then lysed with 100 μl of M-PER lysis buffer with HALT™ protease inhibitors (Pierce Biotechnology, Rockford, IL). The lysates were freeze-thawed 3× at −70°C for 10 minutes, centrifuged at 14,000 × g for 15 minutes to pellet cellular debris, and the supernatants collected. A small sample was set aside for protein quantification. To the remaining sample, 50 μl of 4× loading buffer was added, and the lysates were boiled for 5 minutes. Lysates (50 μg) were resolved on 4–20% sodium dodecyl sulfate (SDS) polyacrylamide gels by electrophoresis (PAGE), under reducing conditions. Resolved proteins were electroblotted onto nitrocellulose membranes and then incubated with blocking buffer (5% skim milk powder and 0.05% Tween 20 in PBS (TBST) at room temperature with agitation for 30 minutes. The membranes were then washed in TBST and incubated with agitation overnight at 4°C with antibodies for tissue factor or ICAM-1 in TBST with 1% skim milk powder. The membranes were washed again in TBST and incubated for 30 minutes with agitation at room temperature with the appropriate horseradish peroxidase conjugated antibodies (Jackson Immunoresearch; West Grove, PA) in TBST with 1% skim milk. The membranes were washed once more in TBST and then incubated for 2 minutes in Super Signal Chemiluminescent substrate (Pierce). Protein bands were visualized using Kodak Biomax light film (Kodak, New Haven, CT). Afterwards, membranes were stripped using a commercial reagent (Restore™ western blot stripping reagent; Pierce), reblocked 1 hour and probed for β-actin expression (diluted 1:5000).
Flow cytometry
Endothelial cells were washed with PBS, and then detached by a brief incubation with Hanks-EDTA (calcium and magnesium-free Hanks, Sigma, St. Louis, MO; EDTA 2.5 mM, pH 7.4). The cells were incubated in 4% paraformaldehyde and fixed at 4°C for 30 minutes. Endothelial cells were then washed in 500 μl of PBS before being stained with antibodies for tissue factor or ICAM-1. After a 1 hour incubation, the cells were washed and incubated with FITC-conjugated secondary antibodies for 1 hour. Cells were then washed in PBS and analyzed by flow cytometry (FACScan, Becton-Dickinson) using WinMDI software (Scripps Research Institute, La Jolla, CA) and collecting 10,000 events for each sample.
Transmission Electron Microscopy
Endothelial cells were seeded at a density of 1 × 105 cells/ml on glass coverslips in 24 well tissue culture plates, and allowed to adhere overnight. Bovine platelets, prepared and treated as described above, were incubated with the endothelial cells for 12 hours. At that point, cell culture media was removed and the endothelial cells were fixed with a 25% v/v of Karnovsky’s solution (2.5% glutaraldehyde, 4% paraformaldehyde) for 10 minutes at room temperature. The coverslips were washed with PBS, stained with 1% osmium tetroxide for 30 minutes, and then dehydrated through a graded series of ethanol washes. The endothelial cells were then embedded in 100% epoxy resin for 5 hours. Following embedding, the glass coverslip was dissolved by hydrofluoric acid, and samples were thin sectioned, stained with uranyl acetate and lead citrate, and examined with a Philips CM120 STEM transmission electron microscope (Phillips Electron Optics, Eindhoven, The Netherlands). Internalized and adherent platelets were quantified by examining endothelial cells at ×2650 magnification. Platelets in direct contact with endothelial cells were recorded as adherent, while platelets that had been fully endocytosed were recorded as internalized. Approximately 50 endothelial cells from at least 5 separate fields were scored for each treatment.
Statistical analysis
An unbalanced one-way analysis of variance (ANOVA) was used to determine if significant variation existed between group means. To perform pair wise comparisons of all the means, we used Tukey’s multiple comparison tests (p < 0.05) in the Prism 4 statistical package (GraphPad, San Diego, CA). The Tukey test is a conservative test because the critical value is an approximation to the exact value.
Results
Characterization of bovine pulmonary artery endothelial cells
Bovine pulmonary artery endothelial cells were isolated as described previously 16. Endothelial cells were characterized by their “cobblestone” morphology, and their expression of CD146 and von Willebrand factor (vWF) as determined by flow cytometry (Data not shown).
Activated platelets induce endothelial cell surface molecule expression
To determine if activated platelets up-regulate endothelial cell adhesion molecules (E-selectin, ICAM-1) and tissue factor, bovine platelets (1 × 108) were incubated with either ADP (10 μM), H. somni (1 × 108) or H. somni LOS (500 ng/ml) for 10 minutes. Previously, we reported that these conditions significantly increased bovine platelet expression of P-selectin, CD40L and FasL. Activated platelets were then fixed in 4% paraformeldehyde (1/10 vol/vol) for 30 minutes, then washed in PBS and incubated with bovine endothelial cells (MOI 10:1). Endothelial cell expression of E-selectin, ICAM-1 and tissue factor was determined by flow cytometry, real-time PCR and western blot analysis.
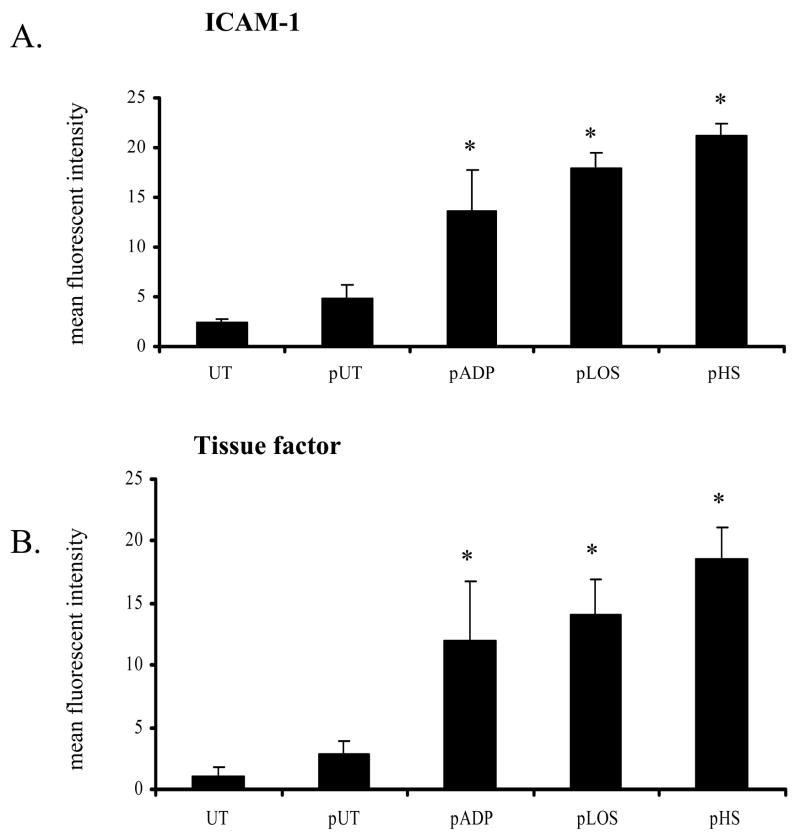
After a four hour incubation with activated platelets, we observed an increase in endothelial cell expression of ICAM-1 and tissue factor as determined by flow cytometry (Figure 1). ICAM-1 and tissue factor expression were significantly increased in endothelial cells incubated with platelets activated by ADP, H. somni LOS or H. somni, compared to control endothelial cells incubated with unactivated platelets (p < 0.05).
Figure 1. Activated platelets induce endothelial cell ICAM-1 and tissue factor expression.
Bovine platelets (1 × 108 cells) were incubated with H. somni (pHS; MOI 1:1) or H. somni LOS (pLOS; 500 ng/ml) for 10 minutes before being briefly fixed in paraformaldehyde. Untreated platelets (pUT) and ADP activated platelets (pADP; 10 μM) were used as negative and positive controls, respectively. Fixed platelets were then washed and incubated with bovine endothelial cells at an MOI of 10 to 1. After four hours, endothelial cells were collected and analyzed by flow cytometry for expression of ICAM-1 (A) or tissue factor (B). The data (A, B) illustrate the mean ± SEM of 3 separate experiments (Tukey’s Test * = p < 0.05 as compared to cells incubated with untreated platelets).
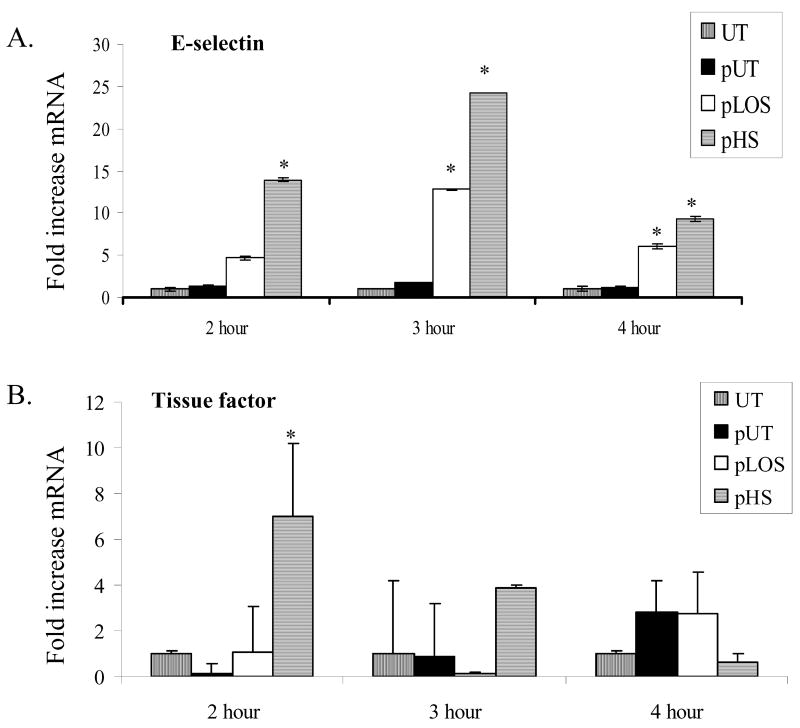
Using reverse-transcriptase real-time PCR, expression of mRNA transcripts for tissue factor and E-selectin were also examined. Endothelial cells incubated with platelets activated by H. somni or H. somni LOS had significantly increased E-selectin mRNA levels at two to four hours of incubation (Figure 2A). H. somni activated platelets also induced significant levels of tissue factor mRNA within 2 hours (Figure 2B).
Figure 2. Activated platelets induce endothelial cell E-selectin and tissue factor mRNA production.
Bovine platelets (1 × 108 cells) were incubated with H. somni (pHS; MOI 1:1) or H. somni LOS (pLOS; 500 ng/ml) for 10 minutes before being fixed in paraformaldehyde. Untreated platelets (pUT) were used as negative a control. Fixed platelets were washed then incubated with bovine endothelial cells at an MOI of 10 to 1. At the indicated time points, endothelial cells were collected and mRNA isolated. Using real-time PCR, transcript levels of mRNA were standardized with our endogenous control (β-actin) and expressed as a fold increase in (A) E-selectin and (B) tissue factor as compared to untreated endothelial cells. These data are the mean ± SEM from one representative experiment of three separate experiments performed (Tukey’s test * = p < 0.05 as compared to cells incubated with untreated platelets).
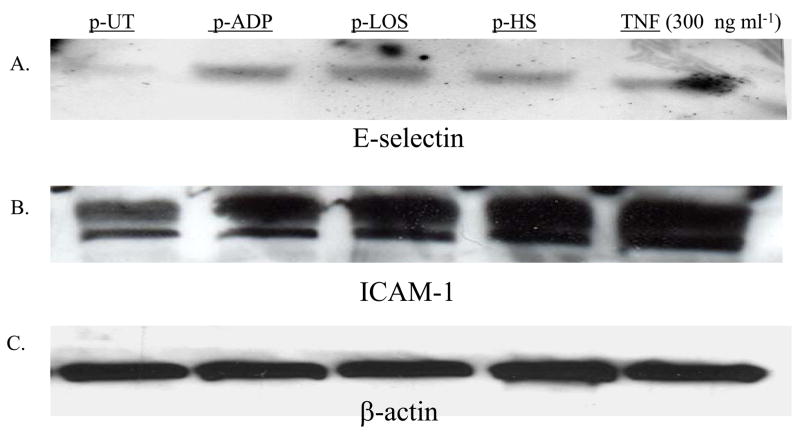
We next measured endothelial cell E-selectin and ICAM-1 protein expression. Following a four hour incubation with activated bovine platelets, endothelial cell lysates were collected and analyzed by PAGE and western blot. Incubation with platelets activated by ADP, H. somni or H. somni LOS increased endothelial cell E -selectin (Figure 3A) and ICAM-1 (Figure 3B) as compared with endothelial cells incubated with unactivated platelets.
Figure 3. Western blot analysis of endothelial cell protein expression of E-selectin and ICAM-1.
Bovine platelets (1 × 108 cells) were incubated with H. somni (pHS; MOI 1:1) or H. somni LOS (pLOS; 500 ng/ml) for 10 minutes before being fixed in paraformaldehyde. Untreated platelets (pUT), and platelets incubated with ADP (pADP; 10 μM), were used as negative and positive controls, respectively. Endothelial cells were also incubated with TNFα (300 ng/ml) in the absence of platelets as an additional positive control. Fixed washed platelets were incubated with endothelial cells at an MOI of 10 to one for four hours at 37°C. The endothelial cells were collected and protein lysates prepared. Approximately 50 μg of endothelial cell lysate were loaded into each lane of a polyacrylamide gel for electrophoresis and analysis by western blotting using antibodies for E-selectin (A), ICAM-1 (B) or β-actin (C). These data are representative of two separate experiments that were performed.
Endothelial cell expression of IL-1β, MCP-1 and MIP-1α following incubation with activated platelets
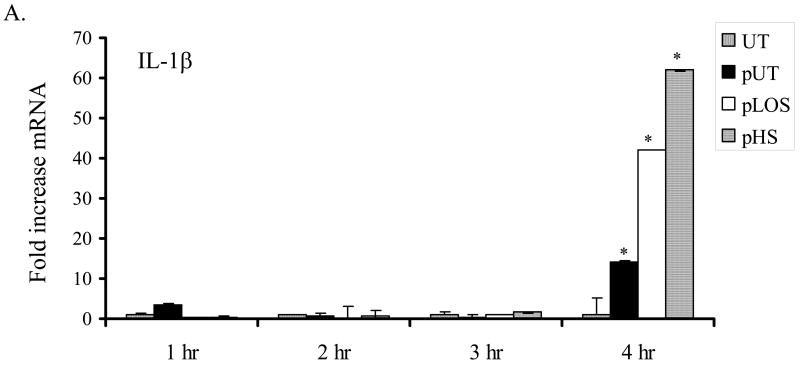
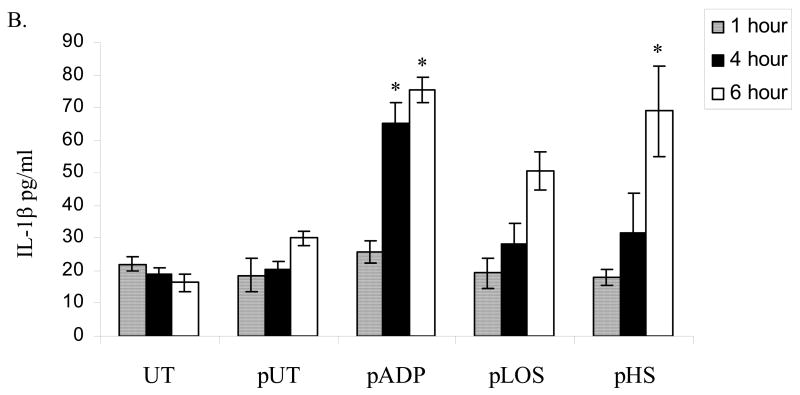
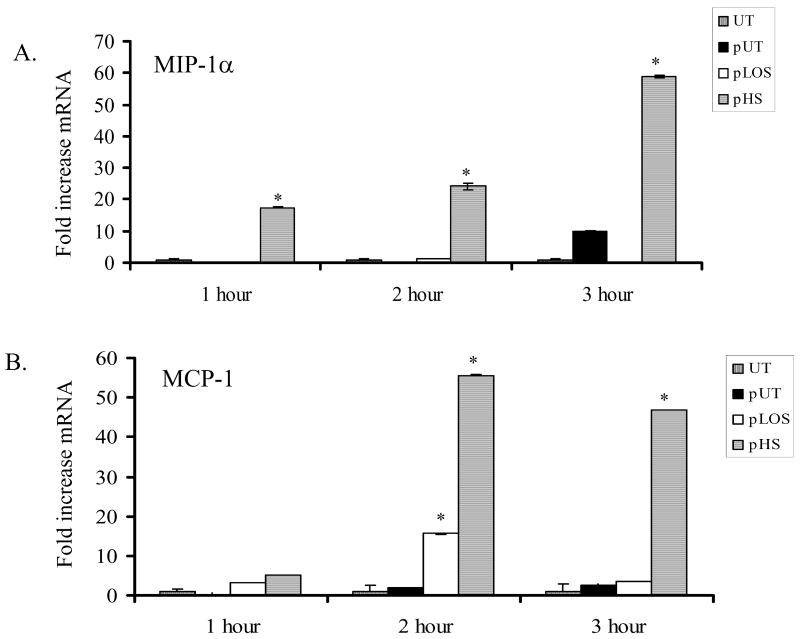
Endothelial cell expression of IL-1β is reported to correlate with the severity of vascular damage during sepsis 23. IL-1β also plays an important role as a pro-inflammatory molecule and promoter of coagulation. We found that endothelial cells incubated for four hours with platelets activated by H. somni or its LOS expressed increased levels of IL-1β mRNA, as compared with untreated cells (Figure 4A). Secretion of IL-1β from endothelial cells was also determined by ELISA. Significant increases in IL-1β protein were detected in conditioned media from endothelial cells incubated for 6 hours with platelets activated by ADP, H. somni, and H. somni LOS, as compared with untreated endothelial cells or cells incubated with unactivated platelets (Figure 4). Bovine endothelial cells incubated for one hour with H. somni activated platelets also exhibited increased MIP-1α (Figure 5A) and MCP-1 (Figure 5), which remained significantly elevated through three hours of incubation. In contrast, H. somni LOS-activated platelets induced a significant increase in endothelial cell MCP-1 mRNA levels only at two hours of incubation (Figure 5).
Figure 4. Incubation with activated bovine platelets increases bovine endothelial cell expression of IL-1β.
Bovine platelets (1 × 108 cells) were incubated with H. somni (pHS; MOI 1:1 ) or H. somni LOS (pLOS; 500 ng/ml) for 10 minutes before being fixed in paraformaldehyde. Untreated platelets (pUT) and ADP treated platelets (pADP; 10 μM) were used as negative and positive controls, respectively. Incubation of endothelial cells with H. somni or LOS activated platelets resulted in increased levels of IL-1β mRNA (A) as assessed by RT-PCR and protein (B) as quantified by ELISA. The data (A) are the mean ± SEM from one representative experiment of three separate experiments performed, while the data (B) illustrate the mean ± SEM of three separate experiments (Tukey’s Test * = p < 0.05 as compared to untreated endothelial cells).
Figure 5. Endothelial cell MIP-1a and MCP-1 mRNA production is induced by activated bovine platelets.
Bovine platelets (1 × 108 cells) were incubated with H. somni (pHS; MOI 1:1) or H. somni LOS (pLOS; 500 ng/ml) for 10 minutes before being fixed in paraformaldehyde. Untreated platelets (pUT) were used as a negative control. The platelets were added to endothelial cells and incubated for 1 to 3 hours at 37°C. Activated platelets induced significant increases in MIP-1α (A) and MCP-1 (B) mRNA expression by one and two hours, respectively. These data are the mean ± SEM from one representative experiment of three separate experiments performed (Tukey’s Test * = p < 0.05 as compared to cells incubated with untreated platelets).
Transmission electron microscopy of endothelial cells interactions with activated platelets
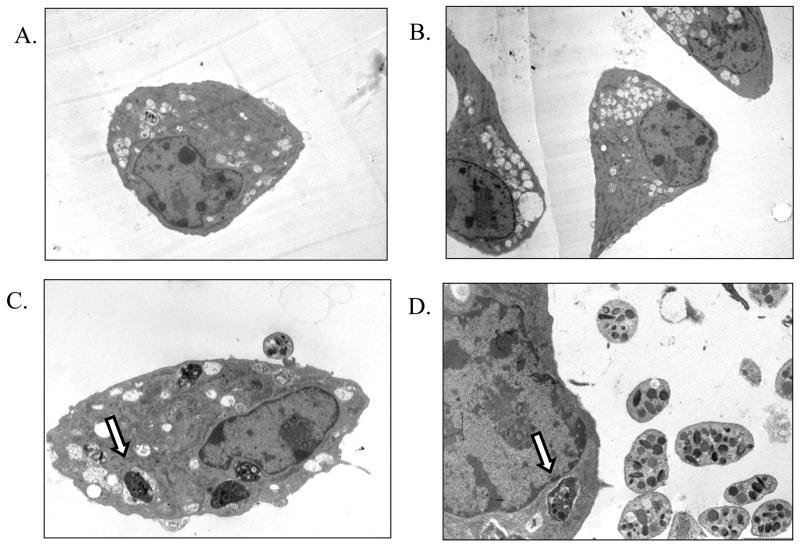
We next used transmission electron microscopy (TEM) to visualize interactions between endothelial cells and platelets. Untreated bovine endothelial cells exhibited the typical polygonal morphology, as did endothelial cells incubated with unactivated platelets (Figure 6A, 6B). We rarely observed direct contact of unactivated platelets with endothelial cells (Figure 6C, 6D). An interesting but infrequent finding were endothelial cells with phagocytized platelets (Figure 6D).
Figure 6. Transmission electron microscopy of endothelial cells incubated with unactivated platelets.
Bovine pulmonary artery endothelial cells were examined by transmission electron microscopy following incubation with unactivated bovine platelets (10 platelets per endothelial cell). Untreated cells demonstrated typical endothelial cell morphology (A, B; ×2650 magnification). Following incubation with unactivated platelets, we observed no significant alterations in endothelial cell morphology (C; ×5560 magnification) and little platelet adherence. We were surprised to observe what appeared to be bovine platelets inside of endothelial cells (C, D; ×7100 magnification) as indicated by the arrow. These photomicrographs are representative of three separate experiments.
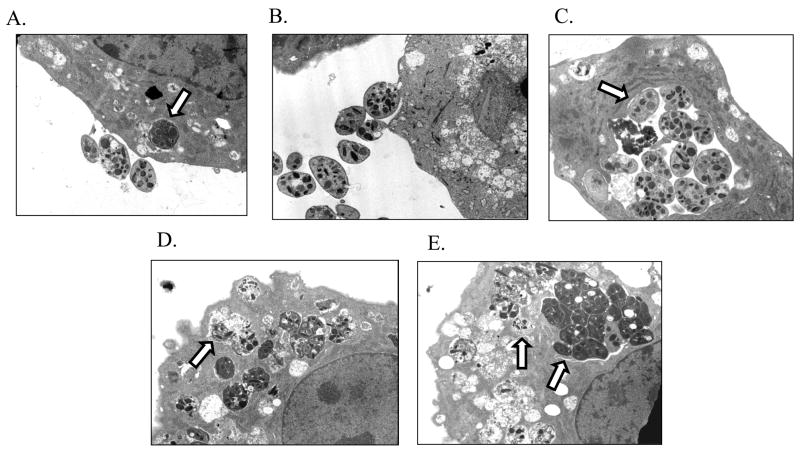
Prior activation of platelets by H. somni LOS resulted in a substantial increase in platelet-endothelial cell adherence (Figure 7A, 7B). We also observed an increase in endothelial cells with internalized platelets (Figure 7A, 7C). Likewise, platelets incubated with ADP formed aggregates, some of which could be found internalized within endothelial cells (Figure 7D). In addition, we found that endothelial cells that had internalized and degraded platelets exhibited ruffled outer membranes (Figure 7D, 7E), suggestive of cell stress or damage.
Figure 7. Transmission electron microscopy of endothelial cell interactions with ADP or LOS activated platelets.
Bovine pulmonary artery endothelial cells were incubated for 12 hours with bovine platelets that had been activated by H. somni LOS (pLOS; 500 ng/ml) or ADP (10 μM). Platelets incubated with H. somni LOS were in close association with endothelial cells (A, B; ×5560 magnification). As indicated by the arrows (B, C) platelets were also found inside of endothelial cells, as were platelets activated with ADP (D, E; ×5650 magnification). In addition, the decomposition of platelets inside of endothelial cells appears to be associated with membrane ruffling suggestive of endothelial cell injury (D, E). These photomicrographs are representative of three separate experiments.
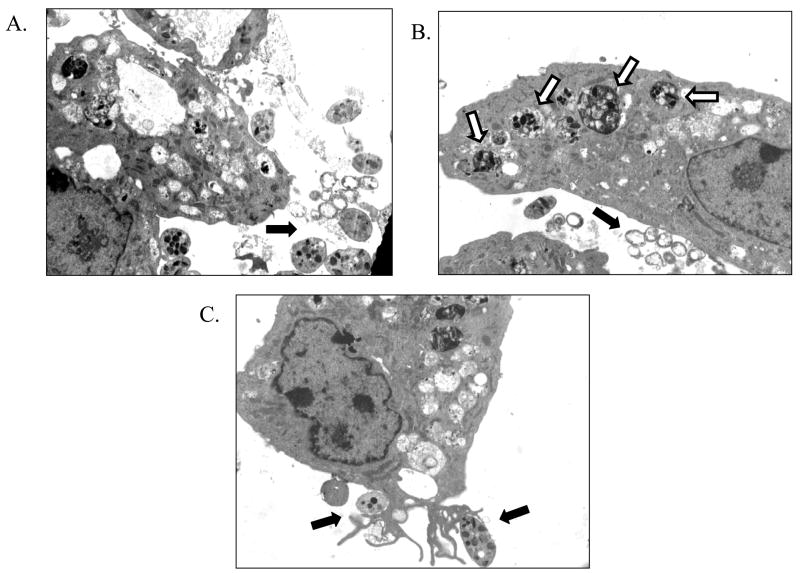
When we incubated bovine endothelial cells with H. somni activated platelets, we observed H. somni in close proximity to both endothelial cells and platelets. Endothelial cells internalized significant numbers of H. somni activated platelets, but not H. somni cells (Figure 8A, 8B). This latter finding confirmed earlier reports, in which H. somni was rarely found internalized by endothelial cells as assessed by TEM 16, 24. One mechanism for the internalization of H. somni activated platelets may be through selective receptor-mediated interactions with endothelial cells leading, to phagocytosis (Figure 8C).
Figure 8. Transmission electron microscopy of endothelial cell interactions with H. somni activated platelets.
Platelets activated by prior incubation with H. somni ( A –C; ×2650 magnification) were found to be in close association with the endothelial cells. Although H. somni cells could be found in close contact with both platelets and endothelial cells (black arrows), they were rarely found inside of endothelial cells. In contrast, endothelial cells appeared to readily ingest activated platelets (A, B; white arrows). An endothelial cell with pseudopods interacting with H. somni-activated platelets is illustrated in (C, black arrows). These photomicrographs are representative of three separate experiments.
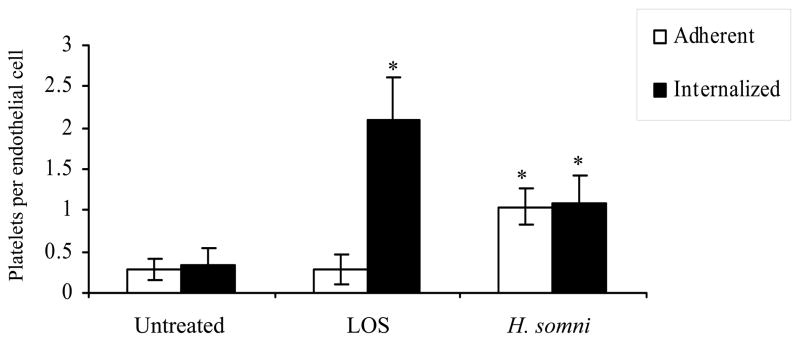
Using transmission electron microscopy the number of H. somni activated platelets adherent to and internalized by bovine endothelial cells was quantified. A small number of endothelial cells endocytosed untreated platelets, and this number was significantly increased in endothelial cells incubated with platelets activated by H. somni or its LOS (Figure 9).
Figure 9. H. somni activated bovine platelets adhere to and are internalized by bovine pulmonary artery endothelial cells.
Using transmission electron microscopy the number of platelets adherent to and internalized by bovine endothelial cells was quantified. Approximately 50 endothelial cells from 5 separate fields were examined. Platelets in direct contact with endothelial cells, but not internalized, were recorded as adherent. Platelets found inside endothelial cells were recorded as internalized. These data are the mean ± SEM of two separate experiments (Tukey’s Test * = p < 0.05 as compared to endothelial cells incubated with untreated platelets).
Discussion
Platelets are important for maintaining haemostasis and promoting inflammation. Activated platelets have been demonstrated previously to up-regulate endothelial cell adhesion molecules and inflammatory cytokine secretion and therefore may play an important role in vasculitis18–20. It has been demonstrated that preventing platelet adherence to the endothelium reduces the severity of endothelial cell damage and vascular inflammation during sepsis 25, 26. In contrast, inhibiting leukocyte adherence had less of an effect. Whether the mechanisms that trigger these events are mediated principally by activated platelets or endothelial cells is not yet known. In this study, we demonstrated that activated bovine platelets induce endothelial cell expression of adhesion molecules (ICAM-1, E-selectin), tissue factor, IL-1β, and chemokines (MCP-1, MIP-1α). A surprising and exciting finding was that bovine pulmonary artery endothelial cells appeared to endocytose activated platelets. This is a novel finding that likely represents endothelial cell recognition of a surface molecule on activated platelets, because nearby bacteria were not phagocytized.
Platelets can be activated directly by bacteria, including several bacterial species linked to sepsis in humans, such as Staphylococcus aureus, Streptococcus pyogenes, and Escherichia coli 27–30. Endotoxin is also known to be a potent activator of platelets 31–34. For example, the activation of human platelets by the LOS of Neisseria meningiditis is thought to be important in microthrombosis and organ dysfunction observed in meningococcal septicemia 35.
We have demonstrated that H. somni activated bovine platelets induced endothelial cell expression of adhesion molecules and cytokines. Some endothelial cell factors are stored as pre-formed proteins in intracellular Weibel-Pallade bodies that are spontaneously released following endothelial cell activation, whereas others factors require active synthesis 36. As a result, we used a variety of methods to detect both surface expression and de novo synthesis. We observed that endothelial cell expression of ICAM-1, E-selectin and tissue factor were significantly up-regulated by H. somni activated platelets. The expression of these molecules in vivo could lead to enhanced leukocyte adherence to the endothelium and promote thrombus formation.
We also observed the production of the chemokines MCP-1 and MIP-1α by endothelial cells incubated with activated platelets. These chemotactic proteins have been reported to be expressed by endothelial cells incubated with activated human platelets, and can promote leukocyte recruitment to areas of inflammation 20, 37. Using real-time PCR, we found both MCP-1 and MIP-1α were significantly up-regulated following endothelial cell incubation with activated platelets. We also examined endothelial cell expression of IL-1β, which can activate pro-inflammatory responses in leukocytes and endothelial cells. We found that platelets activated by H. somni, or its LOS, induced endothelial cell expression of IL-1β, as measured by RT-PCR and ELISA
The most novel and unexpected finding in this study was the engulfment of activated platelets by bovine endothelial cells. Unactivated bovine platelets were rarely internalized by endothelial cells. However, platelets activated by ADP, H. somni or its LOS were internalized at much higher numbers by a greater number of endothelial cells. We found that activated platelets were physically associated with endothelial cells which likely contributes to platelet endocytosis. In addition, internalized platelets exhibited signs of degradation, a process that also appeared to cause morphological changes indicative of cell stress in the endothelial cells. These events may perhaps contribute to the bovine endothelial cell apoptosis that has been previously reported 21. Phagocytosis of platelets by monocytes has been shown to inhibit apoptosis of the latter, however, similar findings have not been reported for endothelial cells 38. In the present study, platelets appeared to be selectively phagocytized, because nearby H. somni cells were not internalized by endothelial cells. This observation confirms earlier reports that bovine endothelial cells rarely phagocytize H. somni 16, 24.
The mechanisms by which bovine endothelial cells phagocytize platelets are unclear at this time. It has been reported that human platelets can fuse their cytoplasic membranes with those of human endothelial cells 39. In that study, platelet granule contents were released as the platelet and endothelial cell membranes merged together. Although a similar mechanism might have occurred in our study, because we used fixed platelets, simple fusion of the platelet cell membrane with that of the endothelial cell seems unlikely due to cross-linking of membrane components. However, human endothelial cells have been reported to phagocytize formaldehyde-fixed human platelets, an event that was associated with an increase in production of reactive oxygen species 40. The mechanism responsible could be mediated by CD40L, P-selectin or other proteins on the platelet surface binding to their cognate receptors on endothelial cells.
The hallmark of systemic H. somni infection in cattle is diffuse vascular inflammation, which on occasion leads to an acute and rapidly fatal cerebral vasculitis known as TME. Activated platelets are known to play critical roles in the pathogenesis of sepsis, by promoting vascular inflammation and coagulation. Previously, we have demonstrated that H. somni and its LOS can activate bovine platelets, and that activated bovine platelets also induce bovine endothelial cell apoptosis. In this study, we have shown that H. somni activated platelets can induce endothelial cell expression of surface molecules (E-selectin, ICAM-1 and tissue factor) IL-1β and chemokines (MCP-1, MIP-1α). We believe that these pro-inflammatory surface molecules and inflammatory mediators may contribute to the pathogenesis of vasculitis that occur during H. somni infection.
Acknowledgments
This work was supported by funding from the University of Wisconsin School of Veterinary Medicine, the Wisconsin Agriculture Experiment Station (Project 3094), the United States Department of Agriculture National Research Initiative 00-35204-9212. We thank Yongjing Li for help in preparing samples for transmission electron microscopy. Thanks to Dr. Mike Howard and Dr. Thomas Inzana at the Virginia Polytechnic Institute for the preparation of H. somni LOS.
References
- 1.Ellis JA. The immunology of the bovine respiratory disease complex. Vet Clin North Am Food Anim Pract. 2001 Nov;17(3):535–550. vi–vii. doi: 10.1016/S0749-0720(15)30005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loneragan GH, Dargatz DA, Morley PS, Smith MA. Trends in mortality ratios among cattle in US feedlots. J Am Vet Med Assoc. 2001 Oct 15;219(8):1122–1127. doi: 10.2460/javma.2001.219.1122. [DOI] [PubMed] [Google Scholar]
- 3.Andrews JJ, Anderson TD, Slife LN, Stevenson GW. Microscopic lesions associated with the isolation of Haemophilus somnus from pneumonic bovine lungs. Vet Pathol. 1985 Mar;22(2):131–136. doi: 10.1177/030098588502200206. [DOI] [PubMed] [Google Scholar]
- 4.Corbeil LB, Arthur JE, Widders PR, Smith JW, Barbet AF. Antigenic specificity of convalescent serum from cattle with Haemophilus somnus-induced experimental abortion. Infect Immun. 1987 Jun;55(6):1381–1386. doi: 10.1128/iai.55.6.1381-1386.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gogolewski RP, Leathers CW, Liggitt HD, Corbeil LB. Experimental Haemophilus somnus pneumonia in calves and immunoperoxidase localization of bacteria. Vet Pathol. 1987 May;24(3):250–256. doi: 10.1177/030098588702400309. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey JD, Little PB, Barnum DA, Doig PA, Stephens LR, Thorsen J. Occurrence of “Haemophilus somnus” in bovine semen and in the prepuce of bulls and steers. Can J Comp Med. 1982 Apr;46(2):215–217. [PMC free article] [PubMed] [Google Scholar]
- 7.Harris FW, Janzen ED. The Haemophilus somnus disease complex (Haemophilosis): A review. Can Vet J. 1984 Oct 1989;30:816–822. [PMC free article] [PubMed] [Google Scholar]
- 8.Corbeil LB, Blau K, Prieur DJ, Ward AC. Serum susceptibility of Haemophilus somnus from bovine clinical cases and carriers. J Clin Microbiol. 1985 Aug;22(2):192–198. doi: 10.1128/jcm.22.2.192-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomis SM, Godson DL, Beskorwayne T, Wobeser GA, Potter AA. Modulation of phagocytic function of bovine mononuclear phagocytes by Haemophilus somnus. Microb Pathog. 1997 Jan;22(1):13–21. doi: 10.1006/mpat.1996.0086. [DOI] [PubMed] [Google Scholar]
- 10.Czuprynski CJ, Hamilton HL. Bovine neutrophils ingest but do not kill Haemophilus somnus in vitro. Infect Immun. 1985 Nov;50(2):431–436. doi: 10.1128/iai.50.2.431-436.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbeil LB, Bastida-Corcuera FD, Beveridge TJ. Haemophilus somnus immunoglobulin binding proteins and surface fibrils. Infect Immun. 1997 Oct;65(10):4250–4257. doi: 10.1128/iai.65.10.4250-4257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inzana TJ, Hensley J, McQuiston J, et al. Phase variation and conservation of lipooligosaccharide epitopes in Haemophilus somnus. Infect Immun. 1997 Nov;65(11):4675–4681. doi: 10.1128/iai.65.11.4675-4681.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruby KW, Griffith RW, Kaeberle ML. Histamine production by Haemophilus somnus. Comp Immunol Microbiol Infect Dis. 2002 Jan;25(1):13–20. doi: 10.1016/s0147-9571(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 14.Howard MD, Cox AD, Weiser JN, Schurig GG, Inzana TJ. Antigenic diversity of Haemophilus somnus lipooligosaccharide: phase-variable accessibility of the phosphorylcholine epitope. J Clin Microbiol. 2000 Dec;38(12):4412–4419. doi: 10.1128/jcm.38.12.4412-4419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behling-Kelly E, Vonderheid H, Kim KS, Corbeil LB, Czuprynski CJ. Roles of cellular activation and sulfated glycans in Haemophilus somnus adherence to bovine brain microvascular endothelial cells. Infect Immun. 2006 Sep;74(9):5311–5318. doi: 10.1128/IAI.00614-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sylte MJ, Corbeil LB, Inzana TJ, Czuprynski CJ. Haemophilus somnus induces apoptosis in bovine endothelial cells in vitro. Infect Immun. 2001 Mar;69(3):1650–1660. doi: 10.1128/IAI.69.3.1650-1660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sylte MJ, Kuckleburg CJ, Inzana TJ, Bertics PJ, Czuprynski CJ. Stimulation of P2X receptors enhances lipooligosaccharide-mediated apoptosis of endothelial cells. J Leukoc Biol. 2005 Jun;77(6):958–965. doi: 10.1189/jlb.1004597. [DOI] [PubMed] [Google Scholar]
- 18.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998 Feb 5;391(6667):591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 19.Danese S, de la Motte C, Sturm A, et al. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003 May;124(5):1249–1264. doi: 10.1016/s0016-5085(03)00289-0. [DOI] [PubMed] [Google Scholar]
- 20.Cha JK, Jeong MH, Bae HR, et al. Activated platelets induce secretion of interleukin-1beta, monocyte chemotactic protein-1, and macrophage inflammatory protein-1alpha and surface expression of intercellular adhesion molecule-1 on cultured endothelial cells. J Korean Med Sci. 2000 Jun;15(3):273–278. doi: 10.3346/jkms.2000.15.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuckleburg CJ, Sylte MJ, Inzana TJ, Corbeil LB, Darien BJ, Czuprynski CJ. Bovine platelets activated by Haemophilus somnus and its LOS induce apoptosis in bovine endothelial cells. Microb Pathog. 2005 Jan;38(1):23–32. doi: 10.1016/j.micpath.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Inzana TJ, Iritani B, Gogolewski RP, Kania SA, Corbeil LB. Purification and characterization of lipooligosaccharides from four strains of “Haemophilus somnus”. Infect Immun. 1988 Nov;56(11):2830–2837. doi: 10.1128/iai.56.11.2830-2837.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters K, Unger RE, Brunner J, Kirkpatrick CJ. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res. 2003 Oct 15;60(1):49–57. doi: 10.1016/s0008-6363(03)00397-3. [DOI] [PubMed] [Google Scholar]
- 24.Thompson KG, Little PB. Effect of Haemophilus somnus on bovine endothelial cell in organ culture. Am J Vet Res. 1981 May;42(5):748–754. [PubMed] [Google Scholar]
- 25.Eipel C, Bordel R, Nickels RM, Menger MD, Vollmar B. Impact of leukocytes and platelets in mediating hepatocyte apoptosis in a rat model of systemic endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004 Jan 8; doi: 10.1152/ajpgi.00275.2003. [DOI] [PubMed] [Google Scholar]
- 26.Sun G, Chang WL, Li J, Berney SM, Kimpel D, van der Heyde HC. Inhibition of platelet adherence to brain microvasculature protects against severe Plasmodium berghei malaria. Infect Immun. 2003 Nov;71(11):6553–6561. doi: 10.1128/IAI.71.11.6553-6561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clawson CC, White JG. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol. 1971 Nov;65(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- 28.Clawson CC, White JG. Platelet interaction with bacteria. II. Fate of the bacteria. Am J Pathol. 1971 Nov;65(2):381–397. [PMC free article] [PubMed] [Google Scholar]
- 29.Clawson CC. Platelet interaction with bacteria. 3. Ultrastructure. Am J Pathol. 1973 Mar;70(3):449–471. [PMC free article] [PubMed] [Google Scholar]
- 30.Clawson CC, Rao GH, White JG. Platelet interaction with bacteria. IV. Stimulation of the release reaction. Am J Pathol. 1975 Nov;81(2):411–420. [PMC free article] [PubMed] [Google Scholar]
- 31.MacIntyre DE, Allen AP, Thorne KJ, Glauert AM, Gordon JL. Endotoxin-induced platelet aggregation and secretion. I. Morphological changes and pharmacological effects. J Cell Sci. 1977 Dec;28:211–223. doi: 10.1242/jcs.28.1.211. [DOI] [PubMed] [Google Scholar]
- 32.Nystrom ML, Barradas MA, Jeremy JY, Mikhailidis DP. Platelet shape change in whole blood: differential effects of endotoxin. Thromb Haemost. 1994 May;71(5):646–650. [PubMed] [Google Scholar]
- 33.Saluk-Juszczak J, Wachowicz B, Kaca W. Stimulatory effects of endotoxin on the platelet secretory process. Microbios. 1999;99(392):45–53. [PubMed] [Google Scholar]
- 34.Saluk-Juszczak J, Wachowicz B, Kaca W. Endotoxins stimulate generation of superoxide radicals and lipid peroxidation in blood platelets. Microbios. 2000;103(404):17–25. [PubMed] [Google Scholar]
- 35.Mirlashari MR, Hagberg IA, Lyberg T. Platelet-platelet and platelet-leukocyte interactions induced by outer membrane vesicles from N. meningitidis. Platelets. 2002 Mar;13(2):91–99. doi: 10.1080/09537100220122448. [DOI] [PubMed] [Google Scholar]
- 36.Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006 May;26(5):1002–1007. doi: 10.1161/01.ATV.0000209501.56852.6c. [DOI] [PubMed] [Google Scholar]
- 37.Gerszten RE, Garcia-Zepeda EA, Lim YC, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999 Apr 22;398(6729):718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 38.Lang D, Dohle F, Terstesse M, et al. Down-regulation of monocyte apoptosis by phagocytosis of platelets: involvement of a caspase-9, caspase-3, and heat shock protein 70-dependent pathway. J Immunol. 2002 Jun 15;168(12):6152–6158. doi: 10.4049/jimmunol.168.12.6152. [DOI] [PubMed] [Google Scholar]
- 39.Lou J, Donati YR, Juillard P, et al. Platelets play an important role in TNF-induced microvascular endothelial cell pathology. Am J Pathol. 1997 Nov;151(5):1397–1405. [PMC free article] [PubMed] [Google Scholar]
- 40.Gorog P, Pearson JD, Kakkar VV. Generation of reactive oxygen metabolites by phagocytosing endothelial cells. Atherosclerosis. 1988 Jul;72(1):19–27. doi: 10.1016/0021-9150(88)90058-5. [DOI] [PubMed] [Google Scholar]