Abstract
AIDS-related Kaposi's sarcoma (AIDS-KS), which is the most prevalent AIDS related cancer, arises in a unique environment characterized by profound immunosuppression in conjunction with sustained immunostimulation. Persistent inflammation and the accompanying increased production of reactive species can promote carcinogenesis by numerous routes including sustained cell proliferation, initiation of nuclear and mitochondrial DNA mutations and induction of a proangiogenic environment. Furthermore, during conditions of continuous inflammation, protein nitration can result in irreversible inactivation of enzymes including the cytoprotective and reactive species degrading enzyme, mitochondrial superoxide dismutase (MnSOD). Because MnSOD serves as a putative tumor suppressor gene in addition to its reactive species inactivating capacities, the loss of MnSOD's cytoprotective functions could markedly facilitate malignant transformation. The purpose of this study was to investigate biochemical and molecular pathways by which reactive species facilitate AIDS-KS pathogenesis. Immunohistochemical studies of AIDS-KS tumors showed intense AIDS-KS lesional cell staining for MnSOD, inducible nitric oxide synthase (NOS 2) and the presence of a cellular ‘fingerprint’ of nitrative stress, 3-nitrotyrosine. Collectively, these results that imply reactive species stress occurs in situ. Similarly, cultured AIDS-KS cells derived from the AIDS-KS tumors contained both MnSOD protein and the ‘high output’ isoform, NOS 2. Co-localization studies established that the mitochondria are a primary site for 3-nitrotyrosine localization and immunoprecipitation/immunoblotting experiments confirmed that MnSOD tyrosine nitration occurs in AIDS-KS cells. Functional SOD assays showed that AIDS-KS cells possess significantly lower MnSOD activity relative to matched control cells; findings which correspond with ongoing MnSOD tyrosine nitration and subsequent inactivation within AIDS-KS cells. These results, which show in situ evidence of reactive species stress within AIDS-KS tumors and functional deficits attributable to nitrative stress in tumor-derived AIDS-KS lesional cells, imply that reactive species are intimately associated with AIDS-KS pathogenesis and provide insights for development of novel strategies for AIDS-KS clinical treatments.
Introduction
AIDS-related Kaposi's sarcoma (AIDS-KS) is a systemic disease with a multifactorial pathogenesis including confounding factors such as HIV viral load, induction of latent human herpes virus 8 (HHV-8), intense immunostimulation and opportunistic infections (1-3). Consequently, the environment in which AIDS-KS arises is unique and is characterized by profound immunosuppression in conjunction with sustained immunostimulation. This immune status perturbation, which results in high circulating levels of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), serves to perpetuate the inflammatory response (4,5). Notably, even during the latter stages of AIDS, there is sparing of host phagocytes such as HIV infected macrophages and neutrophils. This retention of host phagocytes in conjunction with high circulating levels of pro-inflammatory mediators promotes phagocytic activation resulting in high tissue levels of reactive species generated during the anti-microbial oxidative burst (6). Another complicating variable is the fact that HIV infection is often accompanied by significant suppression in antioxidant status. Gil et al. determined that relative to age and gender matched controls, serum and blood samples from HIV+ donors showed a significant reduction in activities of the key antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase, diminished levels of the cytoprotective tripeptide glutathione (GSH), and higher indices of lipid peroxidation and nuclear fragmentation (7). Collectively, these findings suggest that the AIDS-KS milieu is conducive for the development of oxidative and nitrosative stress.
Data that support the contribution of oxidative stress in AIDS-KS pathogenesis has also been accrued at the cellular biochemical level (8-11). In vitro studies conducted by our laboratory show AIDS-KS strains demonstrate enhanced susceptibility to oxidative stress as induced by either physiologically relevant levels of hydrogen peroxide (8,9) or a drug capable of undergoing redox cycling (doxorubicin) (10). AIDS-KS cells also show an acute hyper-responsiveness to a pro-inflammatory cytokine that signals by redox-mediated pathways (TNF-α) (11), and possess an inherent perturbation in their thiol redox status characterized by significantly lower levels of both GSH and the GSSG reductase co-factor, NADPH (8,9). These results imply that due to their impaired antioxidant scavenging capacities and thiol redox perturbations, AIDS-KS cells are inherently susceptible to the deleterious, tumorigenic consequences of oxidative stress.
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) promote tumorigenesis by numerous routes including sustained cell proliferation, initiation of nuclear and mitochondrial DNA mutations and, induction of a proangiogenic environment (12-15). An additional mechanism by which RNS facilitate malignant transformation is by inactivation of key antioxidant and DNA repair proteins via nitration, nitrosation and nitrosylation (16-18). The additions of NO2, NO+ or NO to nucleophiles (processes of nitration, nitrosation and nitrosylation, respectively) are among the major RNS-mediated reactions in biological systems (16-18). One RNS susceptible protein target is the tyrosine residue located at the active site in the mitochondrial based antioxidant enzyme manganese SOD (MnSOD) (19). During conditions of sustained inflammation, protein nitration can result in irreversible inactivation of the cytoprotective enzyme, MnSOD (20,21). The subsequent loss of MnSOD's superoxide (O2•−) scavenging ability disrupts the SOD–NO competitive balance for interactions with O2•−, increases the opportunity for O2•−-NO reactions, and favors production of the highly reactive and potent oxidant, peroxynitrite (ONOO−) (12,17). Since the mitochondria are one of the primary sites of oxidative metabolism, decreased mitochondrial antioxidant capacities convey a major cellular impact. Diminished MnSOD function is permissive for reactive species induced mitochondrial DNA mutations, and also facilitates inactivation of another key antioxidant enzyme, catalase (22). Data that show marked reduction of MnSOD function in several human cancers as well as suppression of the malignant phenotype following MnSOD over-expression, imply that MnSOD may function as a tumor suppressor gene (23-25). Consequently, the loss of MnSOD's cytoprotective activities could markedly facilitate malignant conversion.
While it is well accepted that many malignant cells demonstrate aberrant mitochondrial oxidative metabolism and possess variable levels (most often diminished) of MnSOD activity (26-28), such investigations have not been extended to AIDS-related cancers. The purpose of this study was to investigate biochemical and molecular pathways by which RNS facilitates AIDS-KS pathogenesis. Our data demonstrate that nitrative stress occurs in situ within patients' AIDS-KS lesions and also show that cultured AIDS-KS cells derived from these tumors demonstrate impaired MnSOD functional activity as a consequence of tyrosine nitration. Consequently, our findings provide insights for potential novel AIDS-KS treatment strategies inclusive of antioxidants and NOS inhibitors.
Materials and methods
Immunohistochemical characterization of AIDS-KS tissue biopsies for MnSOD, NOS 2 and 3-nitrotyrosine
Immunohistochemistry was conducted using standard techniques to identify presence and cellular localization of MnSOD, nitric oxide synthase 2 (NOS 2) and 3-nitrotyrosine within biopsies of AIDS-KS tumors. The MnSOD studies used the rabbit anti-MnSOD polyclonal antibody (1:500 dilution, StressGen Biotechnologies, Victoria, BC, Canada), followed by a DAKO LSAB detection kit (DAKO, Carpinteria, CA). The NOS 2 studies employed the rabbit polyclonal antibody anti-NOS 2 antibody (1:500 dilution, BIOMOL Research Laboratories, Plymouth Meeting, PA), followed by the biotinylated goat anti-rabbit secondary antibody (1:200, Vector Laboratories, Burlingame, CA). The 3-nitrotyrosine studies used a rabbit polyclonal anti-nitrotyrosine antibody (2 μg/ml, Upstate Biotechnology, Lake Placid, NY), with a secondary antibody consisting of goat anti-rabbit IgG (Vector Laboratories). For the negative 3-nitrotyrosine control, the anti-nitrotyrosine antibody was incubated with 10 mM nitrotyrosine in phosphate buffered saline for 1 h, and this solution was used instead of the anti-nitrotyrosine polyclonal antibody. Isotypic controls were included for the MnSOD and NOS 2 staining studies.
Isolation and culture of AIDS-KS cells
AIDS-KS cells were isolated from biopsy confirmed tumors of AIDS-KS as described previously (29). These AIDS-KS strains have been shown to possess a normal XY karyotype and show aspects of a transformed phenotype including: high production of ‘AIDS-KS’ growth related cytokines such as IL-6, TNF-α and basic fibroblast growth factor (βFGF), capacity to undergo multiple population doublings (>30) in reduced serum (0.5%) medium, and loss of anchorage dependence (30). Furthermore, relative to matched, non-lesional cells from the AIDS-KS donors, the AIDS-KS spindle cells possess unique biochemical features including reduced cytoprotective enzyme function, increased responsiveness to pro-inflammatory cytokines (11), and reduced tolerance to chemotherapeutic agents that function by redox cycling (10,31). The AIDS-KS cells were cultured at 37°C, 5% CO2 in (complete) medium, which consisted of: M-199 (Gibco, Grand Island, NY), supplemented with 15 mM HEPES, 0.23 mg/ml l-glutamine, 11 μg/ml sodium pyruvate, 90 μg/ml sodium heparin (Sigma, St Louis, MO), 10% heat inactivated fetal bovine serum (Hyclone, Logan, UT) and 5% heat inactivated pooled male human serum (Sigma).
For some experiments, human oral mucosal fibroblasts (age and gender matched with the AIDS-KS donors, obtained from HIV− donors) were also used. The HIV− donor fibroblasts were cultured under identical conditions as the AIDS-KS strains. For selected experiments to determine the specific effects of TNF-α, all cells were cultured in serum-free M-199 medium supplemented with l-glutamine, HEPES and sodium pyruvate (base medium).
Determination of total, cytosolic and mitochondrial cellular SOD activity
Cellular SOD activity was determined by a modification of the method of Flohe and Otting (32) by calculating the rate of inhibition of xanthine/xanthine oxidase reduction of acetylated cytochrome c. SOD, xanthine, xanthine oxidase and acetylated cytochrome c were all purchased from Sigma Chemical Company. The assay was conducted on a DU 7400 Beckman spectrophotometer (550 nm, 25°C) equipped with a Peltier temperature regulating device. A five point SOD standard curve was conducted with each assay. To determine MnSOD activity, the cytosolic (CuZn) SOD inhibitor KCN (2 mM final concentration) was added to the assay mix. Cellular SOD activity was reported in units of activity per milligram of cell protein, with 1 U of SOD activity defined as the amount of enzyme that results in a 50% inhibition of the reduction of acetylated cytochrome c. Cellular protein levels were determined by the Lowry method, using bovine γ-globulins as the standard protein (33).
Determination of AIDS-KS cell expression of the NOS isoforms NOS 2 and NOS 3
Semi-quantitative RT–PCR (using the gel digitizing software Kodak 1D Scientific Imaging Systems, Eastman Kodak Company, Rochester, NY) was conducted on total cellular RNA, using an intron-based primer of glyceraldehyde-3-phosphate dehydrogenase as the housekeeping gene and the NOS primer sequences as reported by Jadeski and Lala (34). After amplification, 10 μl of PCR products were analyzed on a 1.2% agarose gel and stained with ethidium bromide. The size of the amplification products were: 462 (NOS 2), 422 (NOS 3) and 121 bp (GADPH). RNA was isolated under the following experimental conditions: (i) log growth in complete medium, (ii) culture in base medium for 30 h, (iii) culture in base medium for 24 h, followed by an additional 6 h in base medium + 100 U/ml of TNF-α.
Western blot analysis for NOS 2
Aliquots of cell lysate containing 40 mg of protein were analyzed by standard SDS–PAGE. Following membrane transfer, proteins were incubated (4°C, overnight) with rabbit polyclonal antibody anti-NOS 2 antibody (1:2000 dilution, BIOMOL). Membranes were then incubated (1 h) with a goat anti-rabbit IgG H&L secondary antibody conjugated with horseradish peroxidase (1:20 000 BIOMOL). Immunoreactive bands were visualized using ECL Plus Western Blotting Detection System (Amersham Pharmacia Biotech, Piscataway, NJ).
Immunoprecipitation and immunoblotting to determine presence of nitrated proteins
Nitrated proteins were detected in accordance with the protocol recommended by Upstate Biotechnology (www.upstate.com) using mouse monoclonal IgG anti-nitrotyrosine agarose beads (Upstate Biotechnology, Waltham, MA). Approximately 1 μg/μl of cell lysate was added to a slurry of anti-nitrotyrosine agarose conjugate and gently rocked overnight at 4°C. After centrifugation and washing, the beads were resuspended in Laemmli sample buffer, boiled for 5 min, microfuged and the supernate was used for SDS–PAGE analysis. MnSOD western analysis was then conducted using the rabbit polyclonal anti-MnSOD antibody (StressGen Biotechnologies, Victoria, BC, Canada; 0.2 μg/ml). Probed membranes were washed and incubated with a goat anti-rabbit IgG heavy and light chain antibody conjugated with horseradish peroxidase (1:15 000). The membranes were stained by the chemiluminescent ECL-Plus method (Amersham Pharmacia Biotech, Piscataway, NJ), and the immunoreactive signals were visualized by exposure to a radiographic film.
Co-localization studies to determine intracellular 3-nitrotyrosine localization
Cells were cultured on poly-l-lysine coated 8-well Nunc Lab-Tek chamber slides (Nalge Nunc International, Rochester, NY), and experiments were conducted under the following experimental conditions: (i) log growth in complete medium, (ii) 48 h sera deprived (negative control), (iii) 24 h sera deprived, followed by a 24 h challenge with 100 U/ml TNF-α in base (sera-free) medium. Mitochondria were visualized using the mitochondrion-selective probe that discerns actively respiring mitochondria, MitoTracker Red CMXRos (Molecular Probes, Eugene, OR). The cells were incubated with the appropriate medium containing 20 nM Mito Tracker Red CM-H2 XRos at 37°C for 45 min, then fixed in medium containing 3.7% formaldehyde, rinsed with phosphate-buffered saline, permeabilized with 0.2% Triton X-100 for 5 min at room temperature, then stained with rabbit polyclonal anti-nitrotyrosine antibody (Molecular Probes, 5 μg/ml) overnight at 4°C, followed by addition of Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes, H&L, 10 μg/ml, 1 h at room temperature). To confirm specificity of staining, studies were also conducted using an isotypic control (normal rabbit IgG, Vector Laboratories). The same fields were photographed under Alexa 488 (green, 3-nitrotyrosine) and Alexa 579 (red, MitoTracker CMXRos) excitation wavelengths.
Statistical analyses
The two-tailed Mann–Whitney U test was used to evaluate differences in SOD functional activities. Differences of P ≤ 0.05 were considered to be statistically significant.
Results
Biopsies of AIDS-KS tissues show evidence of ongoing reactive species stress in vivo
Results of immunohistochemical studies that identified presence and tissue distribution of MnSOD showed distinct differences between AIDS-KS lesions and other oral mucosal tissues (Figure 1A and B). Clinically and microscopically healthy human oral mucosa showed epithelial predominant MnSOD expression (Figure 1A and B). In normal mucosa, connective tissue MnSOD expression is negligible and restricted to infiltrating phagocytes (Figure 1B) or endothelial cells. In contrast, AIDS-KS biopsies show intense MnSOD expression within the AIDS-KS cell tumor islands that infiltrate throughout the connective tissues (Figure 1A).
Fig. 1.
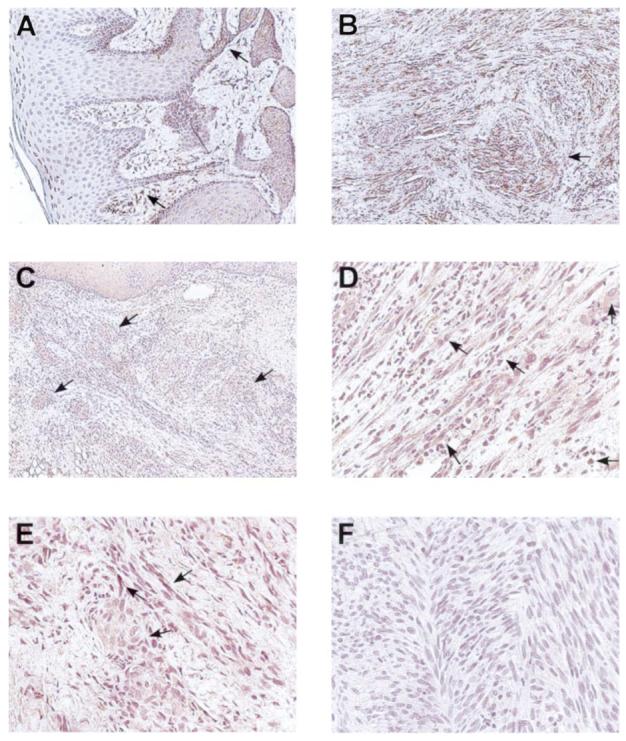
Demonstration of ongoing oxidative and nitrative stress is tissue biopsies of AIDS-KS lesions. Immunohistochemistry was conducted to identify presence and cellular localization of MnSOD, inducible NOS 2 and 3-nitrotyrosine. While MnSOD staining is predominantly found in surface epithelium and infiltrating phagocytes in healthy mucosa (A), AIDS-KS lesional cells, which are contained within the connective tissue stroma, contain abundant MnSOD protein (B). Both aggregates of AIDS-KS tumor cells (C) and individual AIDS-KS spindle cells (D) show intense lesional cell NOS 2 expression. AIDS-KS tumor islands also showed positive staining indicative of tyrosine nitration (E), while the anti-nitrotyrosine negative control (F) shows an absence of staining. (A and B = 100× image scale, C = 40× image scale, D, E and F = 200× image scale.)
NOS 2 immunohistochemistry results showed intense NOS 2 staining within the AIDS-KS lesional cells (Figure 1C and D). Nests of AIDS-KS lesional cells were easily identifiable from the unstained connective tissue stroma (Figure 1C and D). In comparison, the staining of the surface epithelium overlying the AIDS-KS lesional tissues was less intense (Figure 3C, top). As demonstrated in Figure 1D, both the AIDS-KS lesional spindle cells and infiltrating phagocytes displayed strong NOS 2 positivity within the tumor tissues.
Fig. 3.
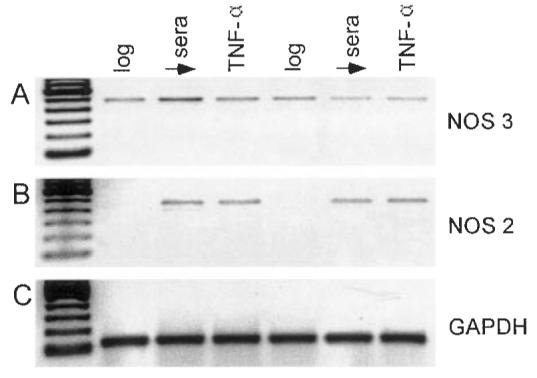
Cultured AIDS-KS cells express both NOS 3 and NOS 2 isoforms. Representative RT–PCR analyses from two AIDS-KS strains confirm the presence of NOS 3 message during all culture conditions. While NOS 2 message was not present during log growth, NOS 2 was expressed following either sera deprivation or TNF-α challenge. (Lanes 1 and 4 = log growth, 2 and 5 = sera deprivation, 3 and 6 = 6 h challenge with 100 U/ml TNF-α.)
Evidence of protein tyrosine nitration and ongoing nitrative stress was noted within the AIDS-KS lesions (Figure 1E) as many AIDS-KS spindle cells and accompanying AIDS-KS tumor nests demonstrated positive 3-nitrotyrosine staining (Figure 1E).
Notably, these immunohistochemical findings were not restricted to selective biopsies from one or two patients. Of the eight AIDS-KS tissues evaluated (selected from clinically advanced nodular AIDS-KS lesions from the oral cavity), all eight biopsies showed AIDS-KS lesional cell staining for MnSOD, NOS 2 and the presence of 3-nitrotyrosine.
Cultured AIDS-KS cells possess significantly lower MnSOD functional activities
Relative to age and gender-matched human oral mucosal fibroblasts, AIDS-KS cells demonstrated comparable levels of cytosolic (CuZn) SOD activity (3.93 ± 1.05 and 3.76 ± 0.45, respectively, n = 6 for each group, mean ± SEM, U/mg protein) (Figure 2). However, AIDS-KS cells showed statistically significantly lower functional activity of mitochondrial (Mn) SOD (P < 0.002, 2.48 ± 0.21 relative to 0.62 ± 0.12; Figure 2). While total SOD activity was reduced in AIDS-KS cells (4.39 ± 0.47 relative to 6.40 ± 0.55), this difference was not statistically significant. As would be anticipated based on the respective MnSOD functional activities, SOD mitochondrial compartmentalization (expressed as a percentage of total SOD activity) was significantly lower in AIDS-KS cells (14.59 ± 7.02 relative to 39.03 ± 2.26, P < 0.002).
Fig. 2.
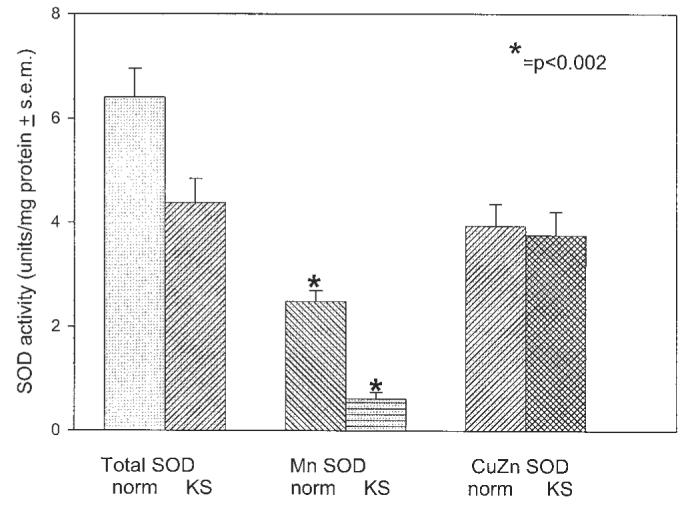
Cultured AIDS-KS cells demonstrate significantly reduced MnSOD functional activity. Cellular SOD activity (units/mg protein) was determined by calculating the rate of inhibition of xanthine/xanthine oxidase reduction of acetylated cytochrome c. Although AIDS-KS cells demonstrated comparable cytosolic (CuZn) SOD function relative to age and gender matched control cells from HIV− donors, AIDS-KS MnSOD activities were significantly decreased.
AIDS-KS cells express both NOS 2 and NOS 3 isoforms and contain NOS 2 protein during in vitro culture
Results of RT–PCR analyses showed that both AIDS-KS cells express both NOS 3 and NOS 2 isoforms during all culture conditions (log growth, sera deprived control and sera deprived control + TNF-α challenge) (Figure 3). As would be anticipated, the stresses of either sera deprivation or inclusion of TNF-α increased expression of the inducible NOS 2 isoform (Figure 3).
Western blot analyses showed that regardless of culture conditions (i.e. log growth in complete medium) sera deprivation (48 h base medium) or TNF-α challenge, cultured AIDS-KS cells contain detectable protein levels of the high output NOS isoform, NOS 2 (Figure 4A).
Fig. 4.
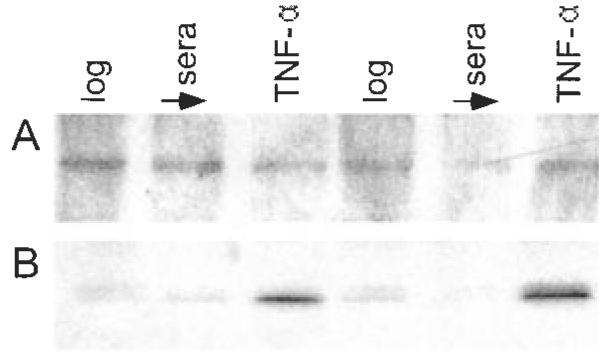
AIDS-KS cells contain NOS 2 protein and demonstrate tyrosine nitration of MnSOD. Western blot analyses (A) showed that regardless of culture conditions, i.e. log growth in complete medium (lanes 1 and 4), sera deprivation (lanes 2 and 5) or TNF-α challenge (lanes 3 and 6), cultured AIDS-KS cells contain detectable protein levels of the high output NOS isoform, NOS 2. Immunoprecipitation-immunoblotting studies showed that AIDS-KS cells demonstrated nitration of their MnSOD tyrosine residues during all culture conditions [log growth (lanes 1 and 4), sera deprived control (lanes 2 and 5), and sera deprived + TNF-α challenge (lanes 3 and 6), B]. Notably, TNF-α challenge markedly increased the extent of MnSOD tyrosine nitration.
MnSOD undergoes tyrosine nitration in AIDS-KS cells
Results of the immunoprecipitation-immunoblotting studies showed that AIDS-KS cells demonstrated nitration of their MnSOD tyrosine residues during all culture conditions (log growth, sera deprived control and sera deprived + TNF-α challenge; Figure 4B). Notably, TNF-α challenge markedly increased the extent of MnSOD tyrosine nitration.
Mitochondria are major intracellular sites of nitrative stress in cultured AIDS-KS cells
Regardless of culture conditions (log, sera deprived control or TNF-α challenge), cultured AIDS-KS cells uniformly showed 3-nitrotyrosine positive staining (Figure 5A and D). Results of the MitoTracker Red CMXRos studies revealed foci of discrete, punctuate intracytoplasmic staining, which is consistent with a mitochondrial localization and retention of mitochondrial oxidative metabolism (Figure 5B, E and H). Co-localization studies (Figure 5C and F) demonstrated that the mitochondria are prime intracellular sites for 3-nitrotyrosine deposition.
Fig. 5.
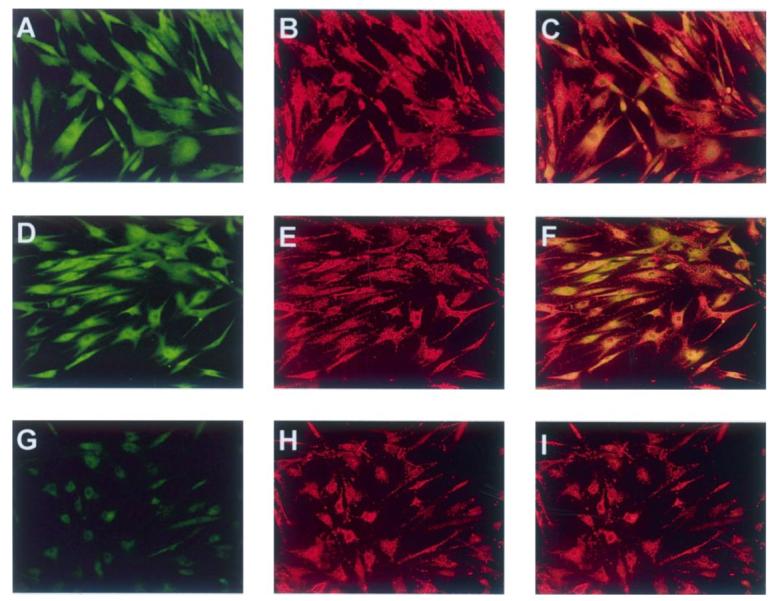
Tyrosine nitration occurs within AIDS-KS mitochondria. Cells were cultured on chamber slides, actively respiring mitochondria localized by addition of MitoTracker Red CMXRos, and the presence of 3-nitrotyrosine confirmed by antinitrotyrosine antibody. The same fields were photographed under Alexa 488 (green, 3-nitrotyrosine) and Alexa 579 (red, MitoTracker CMXRos) excitation wavelengths. Regardless of culture conditions, 3-nitrotyrosine positive staining was observed in the majority of AIDS-KS cells (A, log growth, D, 24 h sera deprivation) and mitochondria were visible as discrete, punctuate intense red staining (B, log growth, E and H, 24 h sera deprivation). Co-localization results reveal foci of yellow-orange staining consistent with 3-nitrotyrosine staining within the mitochondria (C, log growth, F, 24 h sera deprivation, I, 24 h sera deprivation co-localized with isotypic control). (All photomicrographs are at 200× image scale.)
Discussion
AIDS-KS develops in a unique milieu characterized by sustained high levels of pro-inflammatory cytokines and persistent inflammation within a pervasive background of immunosuppression. Such conditions are highly conducive for the subsequent development of reactive species stress. Currently, there are strong data that substantiate the contribution of oxidative stress and persistent inflammation in several human cancers, e.g. Barrett's esophagitis and the subsequent development of esophageal carcinoma, Crohn's disease and colonic adenocarcinoma (6,12-14). However, despite the clinical evidence for ongoing oxidative stress in AIDS patients (7), the contribution of reactive species has not been extended to AIDS-related cancers. Our results, which show in situ evidence of reactive species stress within AIDS-KS tumors and functional deficits attributable to nitrative stress in tumor-derived AIDS-KS lesional cells, imply that reactive species are intimately associated with AIDS-KS pathogenesis and provide insights for development of novel clinical treatments.
AIDS-KS tissue biopsies showed intense lesional cell staining for MnSOD. This increased MnSOD AIDS-KS lesional cell protein detected in situ may appear incongruent with our in vitro data, which show reduced MnSOD function in AIDS-KS cells. However, studies conducted by MacMillan-Crow et al., to investigate the kinetics of nitration during chronic renal rejection (21) also showed high tissue levels of MnSOD protein with concurrent reduction of MnSOD function. The loss of MnSOD functional activity was attributed to tyrosine nitration (21). Similarly, it is probable that much of the MnSOD protein expressed in situ within AIDS-KS lesions is rendered non-functional by tyrosine nitration.
During neoplastic transformation, tissue distribution of NOS and NOS isoform expression may be significantly increased (12-14). Our immunohistochemical results show the presence of NOS 2 protein within AIDS-KS lesional cells in situ, and also demonstrate retention of NOS 2 protein during in vitro culture. While all NOS isoforms generate NO• during the oxidation of l-arginine to citrulline, inducible NOS (NOS 2) is unique. The ‘high output’ NOS 2 isoform is independent of calcium-calmodulin, is up-regulated during inflammation, and most importantly has the capacity to generate substantially higher levels of nitric oxide relative to either NOS 1 or NOS 3 (12,15). Up-regulation of NOS 2 activity in AIDS-KS lesions could augment disease progression by several routes such as induction of angiogenesis, inactivation of cytoprotective and DNA repair enzymes and initiation of mitochondrial and nuclear DNA mutations. Our results also indicated that all AIDS-KS cell strains also expressed NOS 3 (‘endothelial’) NOS during in vitro culture. These findings are consistent with the concept that AIDS-KS arises from a pleuripotent endothelial cell precursor.
Both lesional cells in AIDS-KS biopsies and cultured AIDS-KS cell strains showed positive staining for 3-nitrotyrosine. Notably, 3-nitrotyrosine positivity is not specific for ONOO− generation, as enzyme systems such as myeloperoxidase can generate 3-nitrotyrosine in the absence of NO (18). However, regardless of the responsible nitrating species, e.g. ONOO− or NO2Cl, 3-nitrotyrosine positive staining does indicate the presence of reactive oxygen and nitrogen species, the nitration of protein tyrosine residues, and demonstrates ongoing nitrative stress (18). It is probable that both exogenous and endogenous sources of reactive species stress contribute to AIDS-KS progression. While activated host phagocytes represent a major in vivo exogenous source of RNS/ROI stress, the presence of NOS 2 protein within AIDS-KS cells in situ in combination with our in vitro results, i.e. retention of NOS 2 message and protein and reduced functional MnSOD activity in AIDS-KS cells, also implicates endogenous nitrative stress in AIDS-KS pathogenesis.
Comparable cytosolic (CuZn) SOD functional activities were detected in AIDS-KS cells and in matched cells from HIV− donors. These results compare favorably with other SOD functional analyses performed in human cells (35,36). However, AIDS-KS cells possess significantly lower mitochondrial (MnSOD) activities and mitochondrial enzyme distributions relative to control cells. In conjunction with other cytoprotective enzymes, MnSOD detoxifies reactive oxygen species, which restricts reactive species induced cellular damage. Because MnSOD's cytoprotective roles also include a putative tumor suppressor function, loss of MnSOD function may be exceptionally deleterious and directly facilitate carcinogenesis (23-28). By its abilities to directly interact with both ROS and RNS, SOD also functions as a key regulatory molecule in reactive species chemistry. Recently, we have shown that CuZnSOD and MnSOD modulate nitrosative chemistry in a biphasic and dynamic fashion (37). Therefore, the biological implications of SOD-RNS interactions also encompass modulation of intracellular signaling and modification of the intracellular redox microclimate.
Our immunoprecipitation and immunoblotting analyses demonstrated that MnSOD tyrosine nitration occurs in AIDS-KS cells, and confirmed that TNF-α challenge markedly increased the extent of tyrosine nitration. Complementary co-localization studies revealed that the mitochondria are a primary site for nitration of tyrosine residues as confirmed by the presence of 3-nitrotyrosine. Collectively, the data provide a mechanism to account for diminished MnSOD functional activity in AIDS-KS cells and suggest how reactive species directly contribute to AIDS-KS pathogenesis.
Current therapies for persons with AIDS-KS are primarily palliative (1). However, identification of persons at increased risk to develop AIDS-KS, i.e. HIV positive status combined with elevated HHV-8 titers and suppressed antioxidant status, could provide a targeted population for preventive therapy. Our results, which show evidence of nitrative stress in AIDS-KS tumors, imply that systemically administered antioxidants and NOS inhibitors may be beneficial in AIDS-KS prevention. Notably, recent human clinical trials, which used an orally administered NOS 2 inhibitor in the treatment of asthma, showed the compound was well tolerated and beneficial (38). While oral administration of NOS 2 inhibitors may provide sufficient drug levels to treat incipient lesions, intervention strategies for developed AIDS-KS tumors would probably require both higher intralesional drug levels and multimodal drug therapy. The use of biodegradable poly(lactide-co-glycolide) controlled release delivery vehicles, which can be injected directly into the AIDS-KS lesions to provide high, sustained intratumoral levels without inducing deleterious systemic side effects, would be well suited for such an application (31,39,40).
Acknowledgements
The authors wish to express their appreciation to Dr Leona Ayers, Principal Investigator of the NIH supported AIDS Malignancy Bank, which provided many of the AIDS-KS tissues used in this study. This work was supported by NIH grants CA RO1 95901 and CA UO1 66351. D.J.L. is a recipient of the Ohio Division of the American Cancer Society Fellowship.
Abbreviations
- AIDS-KS
AIDS-related Kaposi's sarcoma
- MnSOD
mitochondrial superoxide dismutase
- NOS
nitric oxide synthase
- RNS
reactive nitrogen species
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor-α
References
- 1.Shah MH, Porcu P, Mallery SR, Caligiuri MA. AIDS-associated malignancies. Cancer Chemother. Biol. Response Modif. 2002;20:633–664. [PubMed] [Google Scholar]
- 2.Jacobson LP, Arnemian HK. An integrated approach to the epidemiology of Kaposi's sarcoma. Curr. Opin. Oncol. 1995;7:450–455. doi: 10.1097/00001622-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Miles D. Pathogenesis of HIV-related Kaposi's sarcoma. Curr. Opin. Oncol. 1994;6:497–502. doi: 10.1097/00001622-199409000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Bailer RT, Lazo A, Ng-Bautista CL, Hout BL, Ness GM, Hegtvedt AK, Blakeslee JR, Stephens RE, Brierley GP, Mallery SR. Comparison of constitutive cytokine release in high and low histologic grade AIDS-related Kaposi's sarcoma cell strains and in sera from HIV+/AIDS-KS+ and HIV+/AIDS-KS-patients. J. Interferon Cytokine Res. 1995;15:473–483. doi: 10.1089/jir.1995.15.473. [DOI] [PubMed] [Google Scholar]
- 5.Breen EC. ‘Pro- and anti-inflammatory cytokines in human immunodeficiency virus infection and acquired immunodeficiency syndrome’. Pharmacol, Ther. 2002;95:295–304. doi: 10.1016/s0163-7258(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 6.Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. 6th Edn. W.B. Saunders; Philadelphia, PA: 1999. pp. 50–88. [Google Scholar]
- 7.Gil L, Martinez G, Gonzalez I, Tarinas A, Alvarez A, Giuliani A, Molina R, Tapanes R, Perez J, Leon OS. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol. Res. 2003;47:217–224. doi: 10.1016/s1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 8.Mallery SR, Ng-Bautista CL, Lantry LE, Ness GM, Hedgvedt AK, Lazo A, Bailer RT, Hout BL, Stephens RE, Brierley GP. Cultured AIDS-related Kaposi's sarcoma cells retain a proliferative bioenergetic profile but demonstrated reduced cytoprotective capabilities. J. Cell Biochem. 1994;56:568–581. doi: 10.1002/jcb.240560418. [DOI] [PubMed] [Google Scholar]
- 9.Mallery SR, Bailer RT, Hohl CM, Ng-Bautista CL, Ness GM, Livingston BE, Hout BL, Stephens RE, Brierley GP. Cultured AIDS-related Kaposi's sarcoma cells demonstrate impaired bioenergetic adaptation to oxidant challenge: implication for oxidant stress in AIDS-KS pathogenesis. J. Cell Biochem. 1995;59:317–328. doi: 10.1002/jcb.240590304. [DOI] [PubMed] [Google Scholar]
- 10.Mallery SR, Clark YM, Ness GM, Minshawi OM, Pei P, Hohl CM. Thiol redox modulation of doxorubicin mediated cytotoxicity in cultured AIDS-related Kaposi's sarcoma cells. J. Cell Biochem. 1999;73:259–277. [PubMed] [Google Scholar]
- 11.Mallery SR, Landwehr DJ, Ness GM, Clark YM, Hohl CM. Thiol redox modulation of tumor necrosis factor-α responsiveness in cultured AIDS-related Kaposi's sarcoma cells. J. Cell Biochem. 1998;68:339–354. doi: 10.1002/(sici)1097-4644(19980301)68:3<339::aid-jcb5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, Mitchell JB. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 13.Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat. Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 14.Burney S, Caulfield JL, Niles JC, Wishok JS, Tannebaum SR. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat. Res. 1999;424:37–49. doi: 10.1016/s0027-5107(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 15.Joshi MS, Ponthier JL, Lancaster JR. Cellular antioxidant and pro-oxidant actions of nitric oxide. Free Rad. Biol. Med. 1999;27:1357–1366. doi: 10.1016/s0891-5849(99)00179-3. [DOI] [PubMed] [Google Scholar]
- 16.Hess DT, Matsumoto A, Nudelman R, Stamler J,S. S-Nitrosylation: spectrum and specificity. Nature Cell Biol. 2001;3:E1–E3. doi: 10.1038/35055152. [DOI] [PubMed] [Google Scholar]
- 17.Miranda KM, Espey MG, Jourd'heuil D, Grisham MB, Fukuto JM, Feelisch M, Wink DA. The chemical biology of nitric oxide. In: Ignarro LJ, editor. Nitric Oxide: Biology and Pathobiology. Academic Press; San Diego, CA: 2000. pp. 3–19. [Google Scholar]
- 18.Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite in vivo? FEBS Lett. 1997;411:157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- 19.MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 20.Macmillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl Acad. Sci. USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacMillan-Crow LA, Cruthrids DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Rad. Biol. Med. 2001;31:1603–1608. doi: 10.1016/s0891-5849(01)00750-x. [DOI] [PubMed] [Google Scholar]
- 22.Grisham MB, Jourd Heuil D, Wind DA. Nitric oxide I. Physiological chemistry of nitric oxide and its metabolites: implications in inflammation. Am. J. Physiol. 1999;276:G315–G321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- 23.Borrello S, Soini Y, Vakkala M, Kahlos K, Paakko P, Kinnula V. MnSOD expression is less frequent in tumour cells of invasive breast carcinomas than in in situ carcinomas or non-neoplastic breast epithelial cells. J. Pathol. 2001;195:156–162. doi: 10.1002/path.946. [DOI] [PubMed] [Google Scholar]
- 24.Weydert C, Roling B, Lui J, Hinkhouse M, Ritchie JM, Oberley LW, Cullen JJ. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol. Cancer Ther. 2003;2:361–369. [PubMed] [Google Scholar]
- 25.Li N, Oberley TD, Oberley LW, Zhong W. Overexpression of manganese superoxide dismutase in DU145 human prostate carcinoma cells has multiple effects on cell phenotype. Prostate. 1998;35:221–133. doi: 10.1002/(sici)1097-0045(19980515)35:3<221::aid-pros8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Borrello S, Soini Y, Vakkala M, Kahlos K, Paakko P, Kinnula V. MnSOD expression is less frequent in tumour cells of invasive breast carcinomas than in in situ carcinomas or non-neoplastic breast epithelial cells. J. Pathol. 2001;195:156–162. doi: 10.1002/path.946. [DOI] [PubMed] [Google Scholar]
- 27.Weydert C, Roling B, Lui J, Hinkhouse M, Ritchie JM, Oberley LW, Cullen JJ. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol. Cancer Ther. 2003;2:361–369. [PubMed] [Google Scholar]
- 28.Duna H, Zhang HJ, Oberley LW, Futscher BW, Bomann FE. MnSOD up-regulates maspin tumor suppressor gene expression in human breast and prostate cancer cells. Antioxid Redox Signal. 2003;5:677–688. doi: 10.1089/152308603770310356. [DOI] [PubMed] [Google Scholar]
- 29.Mallery SR, Ping P, Kang J, Zhu G, Ness GM, Schwendeman SP. Sustained angiogenesis enables in vivo transplantation of mucocutaneous derived AIDS-related Kaposi's sarcoma cells in murine hosts. Carcinogenesis. 2000;21:1647–1653. doi: 10.1093/carcin/21.9.1647. [DOI] [PubMed] [Google Scholar]
- 30.Bailer RT, Lazo A, Ng-Bautista CL, et al. Correlation between AIDS-related Kaposi sarcoma histological grade and in vitro behavior: reduced exogenous growth factor requirements for isolates from high grade lesions. Lymphology. 1995;28:126–137. [PubMed] [Google Scholar]
- 31.Mallery SR, Pei P, Kang J, Ness GM, Ortiz R, Touhalisky JE, Schwendeman SP. Controlled release of doxorubicin from poly(lactide-co-glycolide) microspheres significantly enhances cytoxicity against cultured AIDS-related Kaposi's sarcoma cells. Anticancer Res. 1999;20:2817–2826. [PubMed] [Google Scholar]
- 32.Flohe L, Otting F. Superoxide-dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- 33.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 34.Jadeski LC, Lala PK. Nitric oxide synthase inhibition by NG-nitro-l-arginine methy ester inhibits tumor-induced angiogenesis in mammary tumors. Am. J. Pathol. 1999;155:1381–1390. doi: 10.1016/S0002-9440(10)65240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marklund SL. Regulation by cytokines of extracellular superoxide dismutase and other superoxide dismutase isoenzymes in fibroblasts. J. Biol. Chem. 1992;267:6696–6701. [PubMed] [Google Scholar]
- 36.Stralin B, Marklund SL. Effects of oxidative stress on expression of extracelular superoxide dismutase, CuZn-superoxide dismutase and Mn-superoxide dismutase in human fibroblasts. Biochem. J. 1994;298:347–352. doi: 10.1042/bj2980347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu T-M, Hayton WL, Morse MA, Mallery SR. Dynamic and biphasic modulation of nitrosation reaction by superoxide dismutases. Biochem. Biophys. Res. Commun. 2002;295:1125–1134. doi: 10.1016/s0006-291x(02)00820-3. [DOI] [PubMed] [Google Scholar]
- 38.Hansel TT, Kharitonov SA, Donnelly LE, Erin EM, Currie MG, Moore WM, Manning PT, Recker DP, Barnes PJ. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 2003;17:1298–1300. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- 39.Anderson JH, McArdle SC, Cooke TG. In: Microspheres and Regional Cancer Therapy. Willmott N, Daly JM, editors. CRC Press; Boca Raton: 1994. pp. 57–70. [Google Scholar]
- 40.Mallery SR, Schenderova A, Pei P, Begun S, Ciminieri JR, Wilson RF, Casto BC, Schuller DE, Morse MA. Effects of 10-hydroxycamptothecin, delivered from locally injectable poly(lactide-co-glyclide) microspheres, in a murine human oral squamous cell carcinoma regression model. Anticancer Res. 2001;21:1713–1722. [PubMed] [Google Scholar]


