Abstract
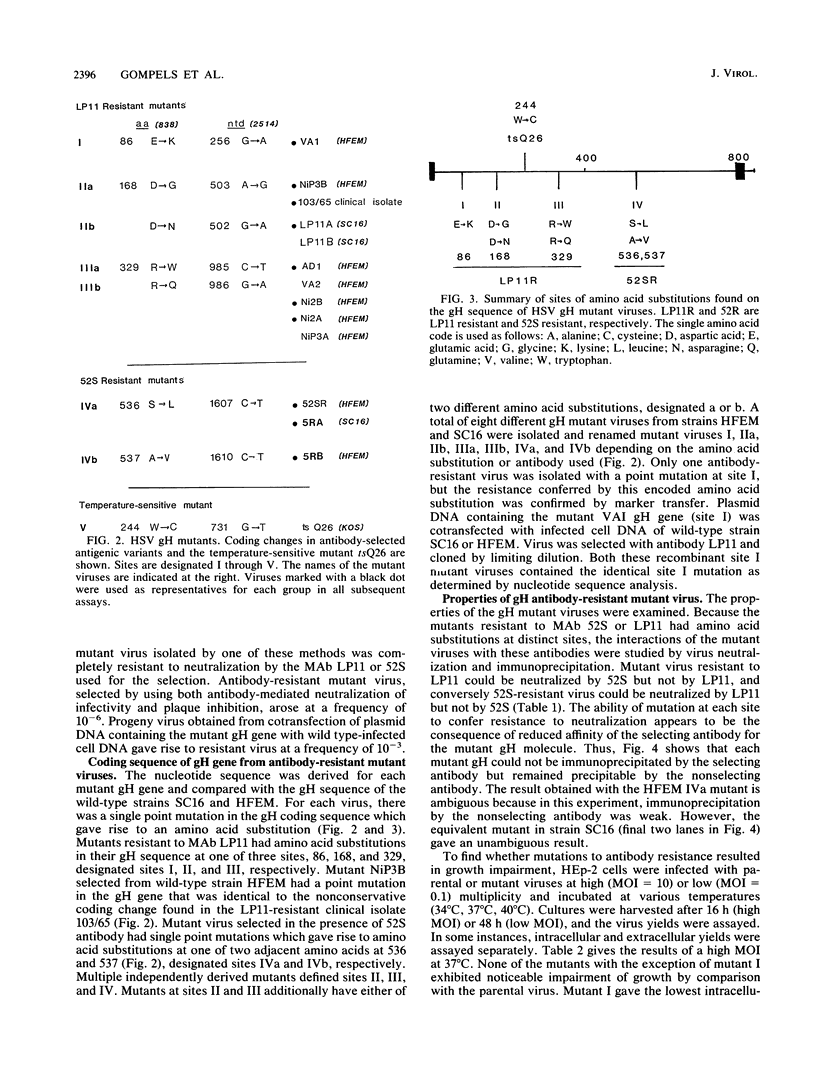
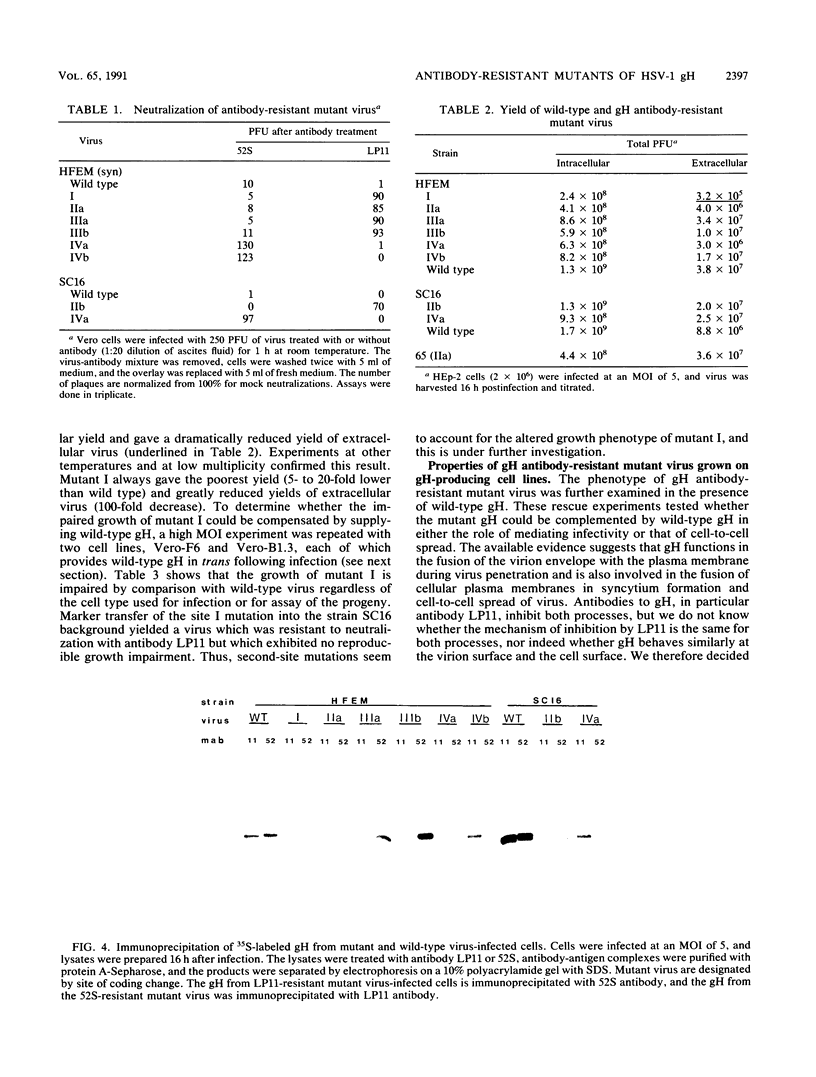
Thirteen antigenic variants of herpes simplex virus which were resistant to neutralization by monoclonal antibody 52S or LP11 were isolated and characterized. The antibodies in the absence of complement potently neutralize infectivity of wild-type virus as well as inhibit the transfer of virus from infected to uninfected cells ("plaque inhibition") and decrease virus-induced cell fusion by syncytial strains. The first variant isolated arose in vivo. Of 66 type 1 isolates analyzed from typing studies of 100 clinical isolates, one was identified as resistant to neutralization by LP11 antibody. The glycoprotein H (gH) sequence was derived and compared with those of wild-type and syncytial laboratory strains SC16, strain 17, and HFEM. The sequences were highly conserved in contrast to the diversity observed between gH sequences from herpesviruses of different subgroups. Only four coding changes were present in any of the comparisons, and only one unique coding change was observed between the laboratory strains and the clinical isolate (Asp-168 to Gly). These sequences were compared with those of antigenic variants selected by antibody in tissue culture. Twelve variants were independently selected with antibody LP11 or 52S from parent strain SC16 or HFEM. For each variant, the gH nucleotide sequence was derived and a point mutation was identified giving rise to a single amino acid substitution. The LP11-resistant viruses encoded gH sequences with amino acid substitutions at sites distributed over one-half of the gH external domain, Glu-86, Asp-168, or Arg-329, while the 52S-resistant mutant viruses had substitutions at adjacent positions Ser-536 and Ala-537. One LP11 mutant virus had a point mutation in the gH gene that was identical to that of the clinical isolate, giving rise to a substitution of Asp-168 with Gly. Both LP11 and 52S appeared to recognize distinct gH epitopes as mutant virus resistant to neutralization and immunoprecipitation with LP11 remained sensitive to 52S and the converse was shown for the 52S-resistant mutant virus. This is consistent with previous studies which showed that while the 52S epitope could be formed in the absence of other virus products, virus gene expression was required for stable presentation of the LP11 epitope, and for transport of gH to the cell surface (Gompels and Minson, J. Virol. 63:4744-4755, 1989). All mutant viruses produced numbers of infectious particles that were similar to those produced by the wild-type virus, with the exception of one variant which produced lower yields.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Longnecker R., Roizman B., Pereira L. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology. 1986 Apr 15;150(1):207–220. doi: 10.1016/0042-6822(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Bell S., Cranage M., Borysiewicz L., Minson T. Induction of immunoglobulin G Fc receptors by recombinant vaccinia viruses expressing glycoproteins E and I of herpes simplex virus type 1. J Virol. 1990 May;64(5):2181–2186. doi: 10.1128/jvi.64.5.2181-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster E. A., Cranage M. P., McLean C. S., Coombs R. R., Minson A. The use of monoclonal antibodies to differentiate isolates of herpes simplex types 1 and 2 by neutralisation and reverse passive haemagglutination tests. J Med Virol. 1984;13(2):193–202. doi: 10.1002/jmv.1890130209. [DOI] [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Cai W. H., Gu B., Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988 Aug;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. Z., Person S., DebRoy C., Gu B. H. Functional regions and structural features of the gB glycoprotein of herpes simplex virus type 1. An analysis of linker insertion mutants. J Mol Biol. 1988 Jun 5;201(3):575–588. doi: 10.1016/0022-2836(88)90639-0. [DOI] [PubMed] [Google Scholar]
- Chapsal J. M., Pereira L. Characterization of epitopes on native and denatured forms of herpes simplex virus glycoprotein B. Virology. 1988 Jun;164(2):427–434. doi: 10.1016/0042-6822(88)90556-9. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Quiroga M., Tung J. W., Dina D., Levy J. A. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990 Sep;64(9):4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier A., Montagnier L., Emerman M. Single amino-acid changes in HIV envelope affect viral tropism and receptor binding. Nature. 1989 Aug 17;340(6234):571–574. doi: 10.1038/340571a0. [DOI] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Smith G. L., Bell S. E., Hart H., Brown C., Bankier A. T., Tomlinson P., Barrell B. G., Minson T. C. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J Virol. 1988 Apr;62(4):1416–1422. doi: 10.1128/jvi.62.4.1416-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Taylor P. Genetic relations between varicella-zoster virus and Epstein-Barr virus. J Gen Virol. 1987 Apr;68(Pt 4):1067–1079. doi: 10.1099/0022-1317-68-4-1067. [DOI] [PubMed] [Google Scholar]
- DeLuca N., Person S., Bzik D. J., Snipes W. Genome locations of temperature-sensitive mutants in glycoprotein gB of herpes simplex virus type 1. Virology. 1984 Sep;137(2):382–389. doi: 10.1016/0042-6822(84)90230-7. [DOI] [PubMed] [Google Scholar]
- Desai P. J., Schaffer P. A., Minson A. C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988 Jun;69(Pt 6):1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- Dowbenko D., Nakamura G., Fennie C., Shimasaki C., Riddle L., Harris R., Gregory T., Lasky L. Epitope mapping of the human immunodeficiency virus type 1 gp120 with monoclonal antibodies. J Virol. 1988 Dec;62(12):4703–4711. doi: 10.1128/jvi.62.12.4703-4711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Santos R. E., Spear P. G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989 Aug;63(8):3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U. A., Craxton M. A., Honess R. W. Conservation of gene organization in the lymphotropic herpesviruses herpesvirus Saimiri and Epstein-Barr virus. J Virol. 1988 Mar;62(3):757–767. doi: 10.1128/jvi.62.3.757-767.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U. A., Craxton M. A., Honess R. W. Conservation of glycoprotein H (gH) in herpesviruses: nucleotide sequence of the gH gene from herpesvirus saimiri. J Gen Virol. 1988 Nov;69(Pt 11):2819–2829. doi: 10.1099/0022-1317-69-11-2819. [DOI] [PubMed] [Google Scholar]
- Gompels U. A., Minson A. C. Antigenic properties and cellular localization of herpes simplex virus glycoprotein H synthesized in a mammalian cell expression system. J Virol. 1989 Nov;63(11):4744–4755. doi: 10.1128/jvi.63.11.4744-4755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Gretch D. R., Kari B., Rasmussen L., Gehrz R. C., Stinski M. F. Identification and characterization of three distinct families of glycoprotein complexes in the envelopes of human cytomegalovirus. J Virol. 1988 Mar;62(3):875–881. doi: 10.1128/jvi.62.3.875-881.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Cai W. H., Person S., Levine M., Glorioso J. C. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J Virol. 1988 Jun;62(6):1881–1888. doi: 10.1128/jvi.62.6.1881-1888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Dorney D. J., Gage P. J., Holland T. C., Cai W., Person S., Levine M., Glorioso J. C. Identification of mar mutations in herpes simplex virus type 1 glycoprotein B which alter antigenic structure and function in virus penetration. J Virol. 1989 Feb;63(2):730–738. doi: 10.1128/jvi.63.2.730-738.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. J., Field H. J., Blyth W. A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975 Sep;28(3):341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- Holtz D., Tanaka R. A., Hartwig J., McKeon F. The CaaX motif of lamin A functions in conjunction with the nuclear localization signal to target assembly to the nuclear envelope. Cell. 1989 Dec 22;59(6):969–977. doi: 10.1016/0092-8674(89)90753-8. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay S., Merigan T. C., Rasmussen L. Identification of cell surface receptors for the 86-kilodalton glycoprotein of human cytomegalovirus. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10100–10103. doi: 10.1073/pnas.86.24.10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousoulas K. G., Huo B., Pereira L. Antibody-resistant mutations in cross-reactive and type-specific epitopes of herpes simplex virus 1 glycoprotein B map in separate domains. Virology. 1988 Oct;166(2):423–431. doi: 10.1016/0042-6822(88)90513-2. [DOI] [PubMed] [Google Scholar]
- Kühn J. E., Kramer M. D., Willenbacher W., Wieland U., Lorentzen E. U., Braun R. W. Identification of herpes simplex virus type 1 glycoproteins interacting with the cell surface. J Virol. 1990 Jun;64(6):2491–2497. doi: 10.1128/jvi.64.6.2491-2497.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Chatterjee S., Whitley R. J., Roizman B. Identification of a herpes simplex virus 1 glycoprotein gene within a gene cluster dispensable for growth in cell culture. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4303–4307. doi: 10.1073/pnas.84.12.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Davison A. J. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 1986 May 27;14(10):4281–4292. doi: 10.1093/nar/14.10.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller N., Hutt-Fletcher L. M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol. 1988 Jul;62(7):2366–2372. doi: 10.1128/jvi.62.7.2366-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson A. C., Hodgman T. C., Digard P., Hancock D. C., Bell S. E., Buckmaster E. A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986 Jun;67(Pt 6):1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- Para M. F., Parish M. L., Noble A. G., Spear P. G. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol. 1985 Aug;55(2):483–488. doi: 10.1128/jvi.55.2.483-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pereira L., Ali M., Kousoulas K., Huo B., Banks T. Domain structure of herpes simplex virus 1 glycoprotein B: neutralizing epitopes map in regions of continuous and discontinuous residues. Virology. 1989 Sep;172(1):11–24. doi: 10.1016/0042-6822(89)90102-5. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Wilson C., Naugle C., Gallo R. C., Robert-Guroff M. Generation of a neutralization-resistant variant of HIV-1 is due to selection for a point mutation in the envelope gene. Cell. 1988 Jul 1;54(1):57–63. doi: 10.1016/0092-8674(88)90179-1. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Buckmaster A., Bell S., Hodgman C., Minson A. C. Identification of a new glycoprotein of herpes simplex virus type 1 and genetic mapping of the gene that codes for it. J Virol. 1986 Feb;57(2):647–655. doi: 10.1128/jvi.57.2.647-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Spear P. G. Membrane proteins specified by herpes simplex viruses. IV. Conformation of the virion glycoprotein designated VP7(B2). J Virol. 1979 Mar;29(3):1159–1167. doi: 10.1128/jvi.29.3.1159-1167.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A., Nash A. A. Role of antibody in primary and recurrent herpes simplex virus infection. J Virol. 1985 Mar;53(3):944–948. doi: 10.1128/jvi.53.3.944-948.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Saxena A., Scott P. I., Song G. J., Probert W. S., Britt W. J., Gibson W., Rasmussen L., Pachl C. Sequence requirements for proteolytic processing of glycoprotein B of human cytomegalovirus strain Towne. J Virol. 1990 Jun;64(6):2922–2931. doi: 10.1128/jvi.64.6.2922-2931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirzaker S. C., Both G. W. The signal peptide of the rotavirus glycoprotein VP7 is essential for its retention in the ER as an integral membrane protein. Cell. 1989 Mar 10;56(5):741–747. doi: 10.1016/0092-8674(89)90677-6. [DOI] [PubMed] [Google Scholar]
- WILDY P., RUSSELL W. C., HORNE R. W. The morphology of herpes virus. Virology. 1960 Oct;12:204–222. doi: 10.1016/0042-6822(60)90195-1. [DOI] [PubMed] [Google Scholar]
- Whealy M. E., Robbins A. K., Enquist L. W. The export pathway of the pseudorabies virus gB homolog gII involves oligomer formation in the endoplasmic reticulum and protease processing in the Golgi apparatus. J Virol. 1990 May;64(5):1946–1955. doi: 10.1128/jvi.64.5.1946-1955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]