Abstract
Background
Resveratrol shows chemopreventative and other biological affects in in vitro and some animal studies. The bioactivities of resveratrol may be attributed to qualitative and quantitative differences in its cell-type-specific interaction and binding with its cellular targets, denoted as resveratrol targeting proteins, RTPs.
Materials and Methods
To isolate RTPs, resveratrol was linked to epoxy-activated agarose generating an affinity platform to allow the isolation, purification, and characterization of distinct RTPs from cultured prostate cancer cell extracts.
Results
Glutathione sulfotransferase-π (GSTP1) and estrogen receptor-β (ER-β) were found to be new RTPs. Resveratrol affinity chromatography was shown to be an easy method for analyzing resveratrol-responsive protein changes in the androgen-dependent LNCaP cells.
Conclusion
Resveratrol affects cellular functions at multiple levels, ranging from interaction with detoxification enzymes, such as GSTP1 and transcription by targeting factors such as ER-β.
Keywords: Biospecific column chromatography, RTPs, GSTP1, ER-β
The demonstration by Doll and Peto in 1981 linking diet and nutrition with causation and prevention of carcinogenesis (1) has prompted efforts to identify diet-based chemopreventive agents. The recent focus has been on dietary polyphenols (2, 3), particularly in regard to their mechanism of action and also the identification of cellular targets that may mediate their bioactivities (4-9).
Resveratrol is a grape-derived polyphenol with chemopreventive and other biological properties. Pezzuto and coworkers have reported that trans-resveratrol inhibited the initiation and promotion of hydrocarbon-induced skin cancer in mice, and the progression of breast cancer in the same species (10). Our own and other studies have shown that resveratrol displayed anti-prostate cancer (CaP) activity via the suppression of cell proliferation, induction of apoptosis, and inhibition of prostate specific antigen (PSA) and androgen receptor (AR) expression in androgen-dependent and hormone refractory CaP cells (11-17).
Resveratrol may exert its anti-CaP effects by binding and interacting with cellular proteins, denoted as resveratrol targeting proteins (RTPs). We have designed a ligand-specific bioaffinity strategy whereby resveratrol was appended to epoxy-activated agarose, creating a platform that captures RTPs for further purification and characterization. This approach has identified quinone reductase 2 (QR2) as a distinct RTP (7, 9). Using the same strategy, cell extracts from different stage CaP cells were investigated and the use of this approach to analyze resveratrol-responsive protein changes in LNCaP cells was also explored.
Materials and Methods
Materials
Epoxy-activated agarose resin and resveratrol were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Resveratrol was dissolved in dimethyl sulfoxide (DMSO) as a 12.5 mM stock solution and kept in aliquots at −20°C.
Cell culture
The LNCaP, CWR22Rv1, PC-3, and DU145 CaP cells representing different stages of CaP were obtained from the American Type Culture Collection (Rockville, MD, USA) and cultured as described previously (7, 11).
Preparation of resveratrol affinity column
Resveratrol was immobilized on epoxy-activated agarose as described previously (7).
Fractionation of cytoplasmic extracts on resveratrol affinity columns
Cytoplasmic extracts from the cultured cells were prepared and fractionated on resveratrol or control columns, as follows. Nonspecifically bound proteins were first removed by washing the column with lysis buffer containing 10 mM Hepes, pH 7.5, 90 mM KCl, 1.5 mM magnesium acetate Mg(OAc)2, 1 mM dithiothreitol (DTT), 0.5% detergent NP-40, 5% glycerol, 10 μl/ml of the protease inhibitor cocktail purchased from Sigma Chemical Co (St. Louis, Mo, USA). The column was sequentially eluted with lysis buffer containing 0.35 M and then 1.0 M NaCl to remove proteins with low affinity for resveratrol. Buffer containing 1 mM ATP was then applied to the columns as a more stringent condition that presumably displaces proteins with higher affinity for resveratrol. Finally, a 1−2 mM resveratrol solution was applied to quantitatively elute proteins avidly bound to the affinity matrix as detailed previously (7). For each condition, the column was eluted 5 times consecutively, with 0.5 ml buffer containing the specified eluant. Controls consisted of mock-treated (immobilization procedure performed on epoxy-activated agarose without addition of resveratrol) or tyrosine-linked column (immobilization on epoxy-activated agarose using tyrosine instead of resveratrol as the ligand). The specificity of protein binding to the affinity resin was assessed by competition, that is by mixing extracts with 1 mM resveratrol prior to fractionation. The proteins displaced using the different elution conditions were concentrated by precipitation using methanol-chloroform-water (18), separated on 10% SDS-PAGE and visualized by silver staining as described previously (7). Identification of RTP-20 from fractionated PC-3 cell extracts by resveratrol affinity chromatography and nano-HPLC-MS/MS. The silver stained band in the resveratrol eluted fraction from the PC-3 cells identified as RTP-20 (see Figure 1A) was excised from the SDS-polyacrylamide gels and completely destained. The gel was treated with 10 mM dithiothreitol in 0.1 M ammonium biocarbonate to reduce the protein. The free cysteine residues were alkylated with freshly prepared 55 mM iodoacetamide dissolved in 0.1 M ammonium bicarbonate buffer. Next, the gel was digested for 16 h at 37°C with agitation by the addition of 25 ng μl−1 Sequence Grade Modified Trypsin (Promega Co., Madison, WI, USA) in ammonium biocarbonate buffer. The digested products were analyzed using nano-liquid chromatography with tandem mass spectrometry (nano-LC-MS/MS). In this method, each trypsin digestion product was first separated and purified by gradient elution with a Dionex capillary/nano-high performance liquid chromatography (HPLC) system (Dionex Corporation, Sunnyvale, CA, USA). The HPLC system consisted of a Dionex Ultimate HPLC pump with Nanoflow Setup, a C18 reverse phase capillary HPLC column and a Dionex Famos autosampler, directly connected to the electrospray source. The peptide mixtures were separated on a self made 15 cm × 75 micron internal diameter reversed-phase C18 column using a 40 min linear gradient of 5−55% acetonitrile in 0.1% formic acid and at a flow rate of 200 nanoL/min. The mass spectra were acquired using a Finnigan LTQ mass spectrometer (Thermo-Fisher, Waltham, MA, USA). The ms/ms spectra were analyzed by a thorough search of the genomic databases using the MASCOT search algorithm (Matrix Science Inc., Boston, MA, USA).
Figure 1.
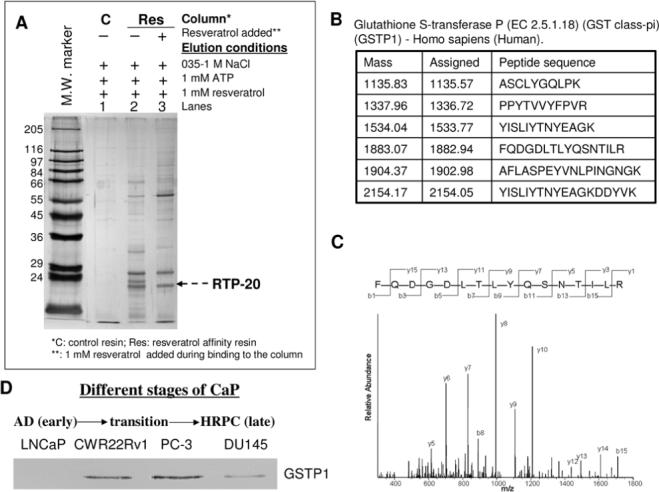
Binding of specific proteins from PC-3 cell extracts to immobilized resveratrol affinity columns and isolation of RTPs. A. Cell lysates fractionated on control and resveratrol affinity columns eluted with NaCl, ATP and resveratrol. Lanes 1−3 show silver stained protein profiles of the resveratrol eluted fraction in control (lane 1) and affinity column with (lane 3) and without (lane 2) competition by resveratrol. Note, there was no evidence of competition between RTP-20 and resveratrol. B. Identification of RTP-20 by tandem mass spectrometry. Six peptide sequences matched the MS/MS spectra, identifying RTP-20 as glutathione sulfotransferase-π (GSTP1). C. A spectrum obtained by tandem MS/MS spectrometry of one of the six peptides listed in (B). D. Differential GSTP1 expression in cell lines representing different stages of CaP, as assayed by Western blot analysis. AD: androgen dependent prostate cancer; HRPC: hormone refractory prostate cancer.
Analysis of control and resveratrol-treated LNCaP cell extracts fractionated by resveratrol affinity chromatography
The LNCaP cells were cultured for 2 days with or without 25 μM resveratrol. Cytoplasmic extracts were prepared and fractionated and the salt, ATP and resveratrol eluted fractions were concentrated, separated on SDS-PAGE and evaluated for the presence of AR, PSA, QR1, QR2, and estrogen receptor-β (ER-β) by Western blot analysis.
Western blot analysis
The expression of specific proteins in the eluted fractions in untreated and resveratrol-treated LNCaP cells and PC-3 cell extracts with and without prior competition with resveratrol was determined by Western blot analysis using the appropriate monoclonal or polyclonal antibodies. As control, the presence of QR2 in various eluted fractions was similarly assayed by Western blot analysis. Enhanced chemiluminescence or color reaction was used to show immunoreactivity (7, 11). The immunoreactive bands were scanned and the mean density of each band was quantified using the ImageJ program (NIH, Bethesda, MD, USA).
Results
Resveratrol affinity chromatography and mass spectrometry
As noted previously (7), most proteins retained on the affinity column were displaced using NaCl or ATP (data not shown), while a few, distinct silver stained protein bands typically were evident in the resveratrol eluates. This was best exemplified by the 20−25-kD triplet in the PC-3 cells (Figure 1A, lane 2). Interestingly, only the middle band, corresponding to a previously identified RTP-22 or QR2 (7, 9), was completely prevented from binding by competition with resveratrol while the binding affinity of the higher and lower migrating bands (designated RTP-20) were little affected by competition using resveratrol (Figure 1A, compare lanes 2 and 3). The same fractionation and analysis using the LNCaP cell extracts showed only two silver stained protein bands in the resveratrol eluted fraction, with the RTP-20 conspicuously absent (data not shown). This notable difference between the LNCaP and PC-3 cells prompted us to further characterize RTP-20. The database search of the MS/MS spectra obtained resulted in the identification of RTP-20 as glutathione sulfotransferase-π (GSTP1) (Figure 1B and 1C).
Western blot analysis showed that while GSTP1 was below detection in the LNCaP cells, it was robustly present in the PC-3 cells, and more modestly and weakly expressed in the CWR22Rv1 and DU145 cells (Figure 1D), which coincided with the respective absence and relative abundance of RTP-20 (data not shown). As reported previously, the DU145 and PC-3 CaP cell lines show expression of GSTP1 while the LNCaP cells are devoid of GSTP1 (22).
Binding and elution of GSTP1 and QR2 on resveratrol affinity columns
Analysis of GSTP1 in fractionated PC-3 cell extracts showed that only about 20% of GSTP1 was retained on the column, while the majority of GSTP1 was found to remain in the unbound lysate. However, the column-bound GSTP1 seemed refractory to elution by NaCl and ATP, and was only displaced from the affinity column by resveratrol (Figure 2). When the resveratrol-competed cell extract was fractionated on the affinity column, a different elution profile was obtained. Namely, small but detectable GSTP1 was continuously eluted by NaCl and ATP, followed by more abundant GSTP1 eluted by the resveratrol. This binding and elution behavior differed strikingly from the quantitative retention of QR2 on the affinity column and its elution by resveratrol (Figure 2) and persisted even when the lysate to affinity resin ratio was altered (data now shown). As expected, the binding of QR2 to the affinity matrix was completely prevented by competition with resveratrol (Figure 2).
Figure 2.
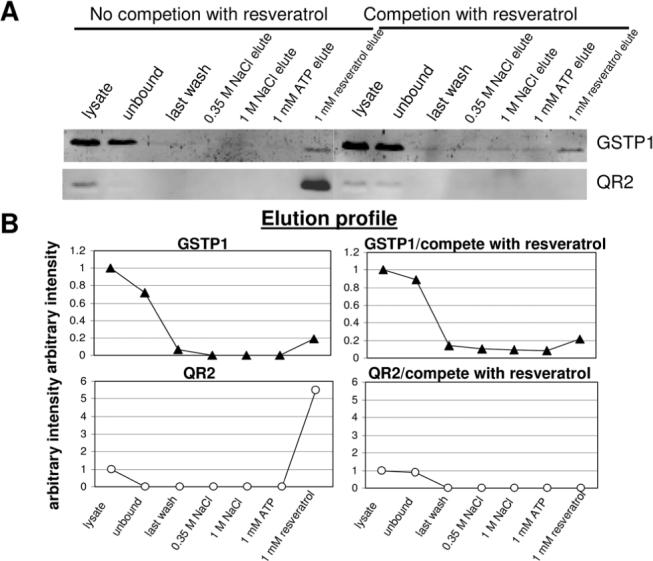
Elution profile of GSTP1 and QR2 in PC-3 cell extracts, with and without prior competition by resveratrol fractionated on resveratrol affinity column. A. Presence of GSTP1 and QR2 in unfractionated, unbound, 0.35 M, 1.0 M NaCl, 1 mM ATP, and 1 mM resveratrol eluted fractions determined by Western blot analysis. B. The immunoreactive GSTP1 and QR2 bands were scanned and the mean density of each band was quantified using the ImageJ program from NIH.
Resveratrol affinity chromatography for analyzing protein level changes in response to treatment with resveratrol
The use of resveratrol affinity chromatography to investigate resveratrol-responsive protein level changes was tested. The lysates from the LNCaP cells, with or without resveratrol treatment, were separately applied to the resveratrol affinity column. The fractions eluted with increasing NaCl, ATP, and resveratrol were analyzed by Western blot for the presence and expression of AR, PSA, QR1, QR2, and ER-β (Figure 3). In the untreated cells, no binding of AR, PSA or QR1 to the affinity matrix was found. In contrast, QR2 was quantitatively retained, as was a fraction of the ER-β, which, curiously, was eluted by NaCl, ATP and resveratrol.
Figure 3.
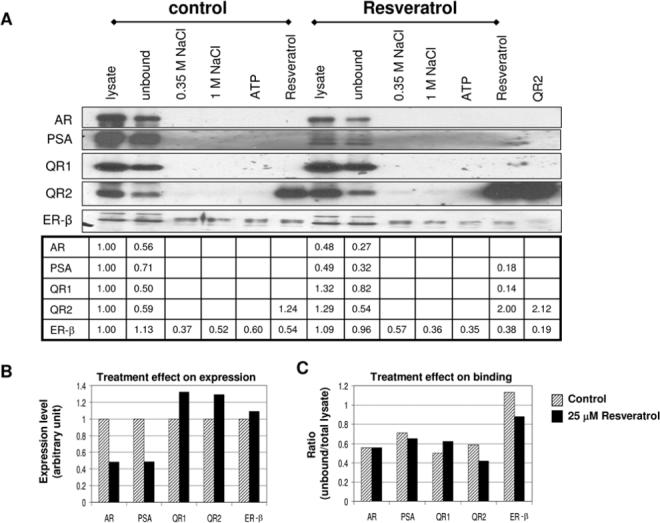
Analysis of protein level changes in response to treatment with resveratrol by resveratrol affinity chromatography. A. Lysates from control and 25 μM resveratrol treated LNCaP cells eluted with NaCl, ATP and 1 mM resveratrol and determined by Western blot analysis. AR: androgen receptor; PSA: prostate specific antigen; QR1, QR2: quinone reductase 1, 2; ER-β: estrogen receptor-β. B. Quantified (in arbitrary units) proteins indicated in panel A. C. The unbound/total (U/T) ratio for the proteins indicated in panel A calculated using arbitrary units.
In the resveratrol-treated cells, a 51% reduction in PSA, matched by a 52% suppression in AR expression, were observed. Treatment with resveratrol increased QR1 and QR2 by 30% and ER-β by 10% (Figure 3A and 3B). The ability of resveratrol to induce QR2 was accentuated using the resveratrol affinity approach, since in the resveratrol eluted fraction of the treated samples, a more pronounced 2-fold elevation of QR2 was evident compared to the similarly fractionated untreated cell extract (Figure 3A). Interestingly, in the fractionated resveratrol-treated cell extracts, PSA (but not AR) and QR1 were detected in the resveratrol eluted fraction. In regard to the specific retention of ER-β on the affinity resin, only a small percentage of the total available ER-β was retained. Again, the elution profile was anomalous, a progressive and continuous displacement from the resin occurred with elution by NaCl, ATP and resveratrol (Figure 3A and 3B).
For each of the proteins, the ratio of unbound to total expression (U/T) was also determined (Figure 3C). For the AR, PSA, and QR1 the U/T ratio between control and treated cells was similar, supporting the observation that there was little to no retention of these proteins on the resveratrol affinity column. However, for QR2, whose total expression increased 30% by resveratrol, a ∼ 30% decrease in U/T ratio was found in the treated cells, confirming the specificity of the affinity column for retaining and concentrating QR2. The U/T ratio for ER-β in the resveratrol-treated cells also showed a decrease, similar to that observed for QR2 and consistent with the observation that more ER-β became bound to the affinity column, as evident by the ∼60% increase in ER-β eluted with 0.35 M NaCl.
Discussion
Cellular responses to resveratrol show a large effective dose range, some cells respond at sub-μM doses (28, 29), while others require logarithmically higher concentrations for efficacy (30, 31). The qualitative and quantitative differences in type and level of RTPs, expressed constitutively and regulated by cellular challenges including chemopreventive agents, might provide a plausible explanation for the broad concentration dependence and diverse bioactivities resveratrol shows in different cells and tissues (7). Using a biospecific affinity approach, QR2 (7), GSTP1 (Figures 1 and 2), and ER-β (Figure 3) have been identified as RTPs. Contrary to the tight binding observed with QR2, binding of GSTP1 and ER-β to the affinity column appears to be weak and heterogeneous, in part supported by the apparent lack of competition of GSTP1 by resveratrol in the PC-3 cell extracts (Figure 1A, lane 3). Preliminary studies suggested that Nrf2 can also bind to a resveratrol affinity column (data not shown). Studies by Davies and coworkers have reported that integrin αVβ3 is a plasma associated target of resveratrol (8). Taken together, these findings provide evidence supporting the notion that resveratrol affects cellular functions at multiple levels, ranging from events at the plasma membrane, to interaction with detoxification enzymes, such as QR2 and GSTP1, and transcription by targeting factors, such as, ER-β and Nrf2 (Figure 4).
Figure 4.
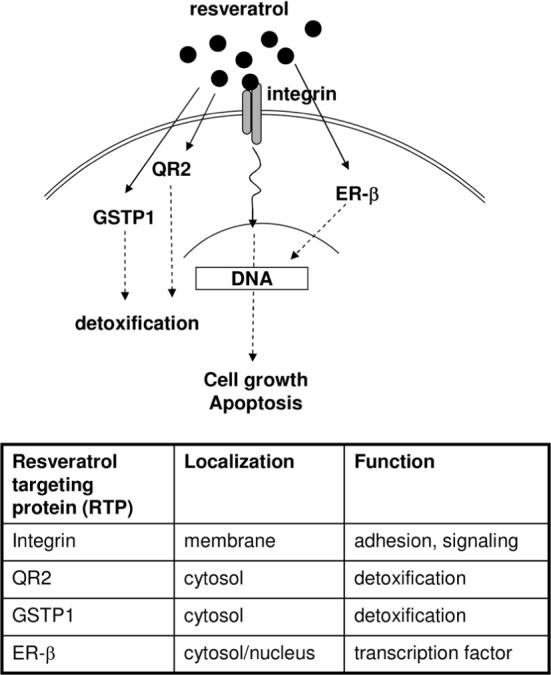
Identity, cellular location, and functions of RTPs. Results from this report and previous studies (7-9) support the hypothesis that resveratrol affects cellular functions at multiple levels, from events at the membrane, to targeting detoxification enzymes QR2 and GSTP1 and modulation of transcription via interaction with ER-β and Nrf2.
The demonstration that GSTP1 is a RTP may be significant in several respects. First, the GSTs, well known for their established role in metabolizing xenobiotics and conferring protection against chemicals with procarcinogenic potential, are also involved in the detoxification of reactive oxygen species (ROS) and thus may well contribute to the maintenance of a redox cellular state (32). Enzymatically, GSTP1 appears to have the highest activity for detoxifying carcinogens (33). Both GSTP1 and GST-α have been clinically correlated with cancer and liver and kidney diseases, while epidemiological studies have linked the polymorphic expression of GSTs with cancer incidence and prognosis (33). Secondly, GST enzymes reportedly can sequester signaling kinases which have an integral though distal role in the control of cell proliferation. For example, GSTP1 physically associates with Jun-JNK (JNK is the c-jun N-terminal kinase capable of phosphorylating and activating the transcription factor c-Jun), resulting in the inhibition of JNK activity (34). Of note, GSTs also actively control post-translational glutathionylation reactions (35). Lastly, human CaP tissue is characterized by a frequent loss of GSTP1 owing to transcriptional silencing via hypermethylation of CG islands in its gene promoter, in high-grade prostatic intraepithelial neoplasia (PIN) and prostate carcinomas (19-21). The absence of GSTP1 may result in less efficient removal of potentially genotoxic compounds, thereby increasing the risk of somatic mutations and tumor transformation. The demonstration that GSTP1 can bind resveratrol may affect the stability of GSTP1, potentially negating some of the adverse consequences associated with the epigenetically driven diminution of GSTP1 activity and function and contributing to the chemopreventive activity of resveratrol in CaP. Whether the stability of QR2 and GSTP1 may be affected by binding to resveratrol is under current investigation in our laboratory.
Some preliminary applications of the resveratrol affinity chromatography approach were also tested in the present study. Conceivably, the affinity platform may enable the identification and profiling of protein level changes elicited in response to treatment with resveratrol. Therefore, the resveratrol-responsive protein expression was tested by analyzing changes in LNCaP cells. Binding of ER-β to the affinity column was not unexpected since resveratrol has been reported to interact with ER, evident by its ability to compete for ER binding in MCF-7 cell extracts and further reinforced by its dose-dependent activation of an estrogen response element driven luciferase reporter gene activity (23-26). A most interesting aspect of these experiments was the U/T ratio analysis for ER-β since, similarly to the observations for QR2, the U/T ratio showed a decrease in the treated cells. This ratio change suggested that more ER-β became bound to the affinity column, which was supported by the ∼60% increase in ER-β eluted with 0.35 M NaCl (Figure 3A). It is tempting to suggest that analysis of the U/T ratio might be indicative of resverestrol-responsiveness for a given protein in terms of its binding to this grape-derived polyphenol, as manifested by retention on the resveratrol affinity column.
Acknowledgements
This research was supported in part by NCI Grant R21CA104424 and by United States Army Prostate Cancer Award W81XWH-04-1-0059 to JMW, and NCI Grant RO3CA109932 to TCH.
References
- 1.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 2.Greenwald P. Cancer chemoprevention. BMJ. 2002;324:714–718. doi: 10.1136/bmj.324.7339.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenwald P, Milner JA, Anderson DE, McDonald SS. Micronutrients in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:217–230. doi: 10.1023/a:1021202709003. [DOI] [PubMed] [Google Scholar]
- 4.Greenwald P. A favorable view: progress in cancer prevention and screening. Recent Results Cancer Res. 2007;174:3–17. doi: 10.1007/978-3-540-37696-5_1. [DOI] [PubMed] [Google Scholar]
- 5.Russo GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem Pharmacol. 2007;74:533–544. doi: 10.1016/j.bcp.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137:223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Hsieh TC, Zhang Z, Ma Y, Wu JM. Identification and purification of resveratrol targeting proteins using immobilized resveratrol affinity chromatography. Biochem Biophys Res Commun. 2004;323:743–749. doi: 10.1016/j.bbrc.2004.08.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin HY, Lansing L, Merillon JM, Davis FB, Tang HY, Shih A, Vitrac X, Krisa S, Keating T, Cao HJ, Bergh J, Quackenbush S, Davis PJ. Integrin alphaVbeta3 contains a receptor site for resveratrol. FASEB J. 2006;20:1742–1744. doi: 10.1096/fj.06-5743fje. [DOI] [PubMed] [Google Scholar]
- 9.Buryanovskyy L, Fu Y, Boyd M, Ma Y, Hsieh TC, Wu JM, Zhang Z. Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry. 2004;43:11417–11426. doi: 10.1021/bi049162o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh TC, Wu JM. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp Cell Res. 1999;249:109–115. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh TC, Wu JM. Grape-derived chemopreventive agent resveratrol decreases prostate-specific antigen (PSA) expression in LNCaP cells by an androgen receptor (AR)-independent mechanism. Anticancer Res. 2000;20:225–228. [PubMed] [Google Scholar]
- 13.Narayanan BA, Narayanan NK, Re GG, Nixon DW. Differential expression of genes induced by resveratrol in LNCaP cells: p53-mediated molecular targets. Int J Cancer. 2003;104:204–212. doi: 10.1002/ijc.10932. [DOI] [PubMed] [Google Scholar]
- 14.Jones SB, DePrimo SE, Whitfield ML, Brooks JD. Resveratrol-induced gene expression profiles in human prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 2005;14:596–604. doi: 10.1158/1055-9965.EPI-04-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayanan NK, Narayanan BA, Nixon DW. Resveratrol-induced cell growth inhibition and apoptosis is associated with modulation of phosphoglycerate mutase B in human prostate cancer cells: two-dimensional sodium dodecyl sulfatepolyacrylamide gel electrophoresis and mass spectrometry evaluation. Cancer Detect Prev. 2004;28:443–452. doi: 10.1016/j.cdp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Gao S, Liu GZ, Wang Z. Modulation of androgen receptordependent transcription by resveratrol and genistein in prostate cancer cells. Prostate. 2004;59:214–225. doi: 10.1002/pros.10375. [DOI] [PubMed] [Google Scholar]
- 17.Lin HY, Shih A, Davis FB, Tang HY, Martino LJ, Bennett JA, Davis PJ. Resveratrol induced serine phosphorylation of p53 causes apoptosis in a mutant p53 prostate cancer cell line. J Urol. 2002;168:748–755. [PubMed] [Google Scholar]
- 18.Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 19.Moskaluk CA, Duray PH, Cowan KH, Linehan M, Merino MJ. Immunohistochemical expression of pi-class glutathione S-transferase is down-regulated in adenocarcinoma of the prostate. Cancer. 1997;79:1595–1599. doi: 10.1002/(sici)1097-0142(19970415)79:8<1595::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Lin X, Tascilar M, Lee WH, Vles WJ, Lee BH, Veeraswamy R, Asgari K, Freije D, van Rees B, Gage WR, Bova GS, Isaacs WB, Brooks JD, DeWeese TL, De Marzo AM, Nelson WG. GSTP1 CpG island hypermethylation is responsible for the absence of GSTP1 expression in human prostate cancer cells. Am J Pathol. 2001;159:1815–1826. doi: 10.1016/S0002-9440(10)63028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins TG, Burns PA, Routledge MN. DNA methylation of GSTP1 as biomarker in diagnosis of prostate cancer. Urology. 2007;69:11–16. doi: 10.1016/j.urology.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Asgari K, Putzi MJ, Gage WR, Yu X, Cornblatt BS, Kumar A, Piantadosi S, DeWeese TL, De Marzo AM, Nelson WG. Reversal of GSTP1 CpG island hypermethylation and reactivation of pi-class glutathione S-transferase (GSTP1) expression in human prostate cancer cells by treatment with procainamide. Cancer Res. 2001;61:8611–8616. [PubMed] [Google Scholar]
- 23.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141:3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 25.Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 26.Gehm BD, Levenson AS, Liu H, Lee EJ, Amundsen BM, Cushman M, Jordan VC, Jameson JL. Estrogenic effects of resveratrol in breast cancer cells expressing mutant and wildtype estrogen receptors: role of AF-1 and AF-2. J Steroid Biochem Mol Biol. 2004;88:223–234. doi: 10.1016/j.jsbmb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell SH, Zhu W, Young CY. Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Cancer Res. 1999;59:5892–5895. [PubMed] [Google Scholar]
- 28.Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol. 2003;64:1029–1036. doi: 10.1124/mol.64.5.1029. [DOI] [PubMed] [Google Scholar]
- 29.Shih A, Zhang S, Cao HJ, Boswell S, Wu YH, Tang HY, Lennartz MR, Davis FB, Davis PJ, Lin HY. Inhibitory effect of epidermal growth factor on resveratrol-induced apoptosis in prostate cancer cells is mediated by protein kinase C-alpha. Mol Cancer Ther. 2004;3:1355–1364. [PubMed] [Google Scholar]
- 30.Haworth RS, Avkiran M. Inhibition of protein kinase D by resveratrol. Biochem Pharmacol. 2001;62:1647–1651. doi: 10.1016/s0006-2952(01)00807-3. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigue CM, Arous N, Bachir D, Smith-Ravin J, Romeo PH, Galacteros F, Garel MC. Resveratrol, a natural dietary phytoalexin, possesses similar properties to hydroxyurea towards erythroid differentiation. Br J Haematol. 2001;113:500–507. doi: 10.1046/j.1365-2141.2001.02746.x. [DOI] [PubMed] [Google Scholar]
- 32.Sato K. Glutathione transferases as markers of preneoplasia and neoplasia. Adv Cancer Res. 1989;52:205–255. doi: 10.1016/s0065-230x(08)60214-6. [DOI] [PubMed] [Google Scholar]
- 33.McIlwain CC, Townsend DM, Tew KD. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006;25:1639–1648. doi: 10.1038/sj.onc.1209373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, Ronai Z. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsend DM, Findlay VJ, Fazilev F, Ogle M, Fraser J, Saavedra JE, Ji X, Keefer LK, Tew KD. A glutathione S-transferase pi-activated prodrug causes kinase activation concurrent with S-glutathionylation of proteins. Mol Pharmacol. 2006;69:501–508. doi: 10.1124/mol.105.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]


