Abstract
Purpose
The objective of this study was to develop poly(lactic-co-glycolic acid) (PLGA) injectable implants (i.e., millicylinders) with microencapsulated N-acetylcysteine (NAC) for site-specific controlled NAC release, for potential chemopreventive applications in persons with previously excised head and neck cancers.
Methods
PLGA 50:50 (i.v.=0.57 dl/g) implants with 1–10 wt% NAC free acid or 10 wt% NAC salts (NAC–Na+, NAC–Mg2+ and NAC–Ca2+) were prepared by solvent extrusion and/or fluid energy micronization (FEM) methods. X-ray diffraction (XRD), scanning electron microscopy (SEM), and differential scanning calorimetry (DSC) studies were performed to evaluate the physical mixing of NAC with PLGA. PLGA implant degradation was studied by kinetics of polymer molecular weight decline (gel permeation chromatography) and mass loss. Release studies were conducted in N2 purged PBS (pH 7.4) at 37°C in evacuated and sealed ampoules. NAC was quantified by HPLC at 210 nm.
Results
XRD, SEM and DSC studies indicated that NAC had dissolved in the polymer phase at 1–3.5% w/w loading, but became discretely suspended in the polymer at 6–10% w/w. Initial burst and long-term release rate increased with increased drug loading, and release was uncharacteristically rapid at higher loading (6–10% w/w). The cause of the rapid release was linked to extensive plasticization, matrix porosity and general acid catalysis of PLGA degradation caused by the NAC free acid. PLGA millicylinders loaded with 10% w/w NAC–Ca2+ and NAC–Mg2+salts exhibited reduced burst (34 vs 13–22% release within a day of incubation for NAC free acid vs NAC–Ca2+ and NAC–Mg2+salts, respectively) and slow and continuous complete release over 4 weeks without significant NAC-catalyzed degradation of PLGA. Release of NAC from NAC–Ca2+/PLGA implant was slower than that of NAC–Mg2+/PLGA consistent with the lower solubility of the former salt. NAC with its free thiol was rapidly converted to its cystine dimer in the presence of molecular oxygen. PLGA released samples in sealed and evacuated ampoules indicated >80% parent NAC remaining after the 1 month release analysis irrespective of initial NAC free acid and salt forms.
Conclusion
By encapsulating the NAC–Mg2+ and NAC–Ca2+ salts in PLGA implants, the high initial burst, short release duration, and the general acid catalysis caused by the NAC free acid were each prevented and 1-month slow and continuous release was attained with minimal instability of the free thiol group.
Keywords: burst effect, controlled release, drug induced plasticization, drug induced PLGA degradation, head and neck cancer, N Acetylcysteine, NAC salts, PLGA degradation, PLGA implants
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the sixth most prevalent cancer worldwide and it is estimated that HNSCC will affect over 34,000 Americans in 2007 (1). Current treatments for HNSCC, which entail wide surgical excision, with or without adjuvant radiation therapy, result in significant morbidity due to loss of tissues vital for esthetics and function. Furthermore, over 50% of HNSCCs recur despite aggressive treatment, which makes local recurrence the most common cause of treatment failure (2). Conventional strategies to prevent local HNSCC recurrence (inclusive of radiotherapy, combined radiation and chemotherapy, immunotherapy and gene therapy) have proven ineffective (2,3). In addition, as avascular scar tissue frequently forms as a post-surgical complication at the tumor resection site, systemic delivery methods dependent on high levels of tissue perfusion are often ineffective (3). The ideal treatment for HNSCC is to prevent its development by intervention during precancerous stages. A promising approach to suppress HNSCC tumor recurrence and the development of second primary tumors even at fibrotic sites is the use of polymeric delivery vehicles capable of providing tissue site specific controlled release of cancer preventing compounds. Our goal is to employ injectable polymeric vehicles that would be placed in the superficial connective tissue at the site of head and neck cancer resection, where they would be expected to provide a therapeutic advantage i.e., high local levels of the therapeutic agent without systemic complications (4-8).
Disruption of matrix metalloproteinase (MMP) regulation results in inappropriate matrix degradation, and facilitates progression of several human diseases, including cancer (9,10). Not surprisingly, increased MMP activity has been detected in a variety of human epithelial derived cancers including colorectal, breast, and oral cavity (11-13). Inappropriately sustained MMP activation enables invasion of the basement membrane by tumor cells (enabling carcinoma-in-situ to progress to overt cancer) and also permits cancer cell invasion into host blood vessels, providing a transport system for distant tumor metastases (12). Head and neck cancer represents one such circumstance when invasion of the basement membrane, resulting in progression of CA-in-situ to invasive cancer, can dramatically change the necessary treatment and eventual clinical outcome. In vivo, MMPs are released as inactive pro-forms. MMP-9, which degrades collagen type IV, is a key enzyme involved with basement membrane degradation and subsequent progression of precancerous oral epithelial lesions to overt cancer (11,13). Our labs have recently shown that as a result of its reduced thiol, NAC: (1) inhibits activation of the proform of MMP-9 (an MMP that is involved in degradation of collagen type IV—a key component of the basement membrane), and (2) serves as a surrogate propeptide and inhibits function of the previously activated MMP-9, putatively by blocking the enzyme's active site (14). The reduced nonprotein thiol-MMP interactions have functional consequences as shown by inhibition of invasion of cultured HNSCC cells following introduction of NAC (14). Due to the importance of extracellular matrix degradation in tumor associated angiogenesis and tumor cell invasion, PLGA injectable implants that provide sustained therapeutic levels of demonstrated MMP inhibitors, NAC for example, have the potential to markedly inhibit locoregional HNSCC tumor recurrence and deter the formation of second HNSCC primary tumors.
NAC is a thiol-containing, mucolytic agent and a precursor of l-cysteine and reduced glutathione (15). The ability of NAC to effectively scavenge free radicals, which are byproducts of normal metabolic activities (16), make this molecule a powerful antioxidant. In addition to cytoprotection, these antioxidant properties contribute to NAC's ability to inhibit proMMP activation and suppress mature enzyme function. Our results, which demonstrate that only the reduced, thiol containing forms of glutathione and NAC inhibit proMMP activation and suppress MMP-9 activity (14), emphasize the need for polymer microclimate regulation in order to preserve thiol integrity.
The objective of present study was to develop injectable PLGA implant (millicylinders) which can slowly and continuously release NAC for potential application in head and neck cancer. The effect of formulation parameters such as NAC loading and free acid vs. salt form (NAC–Mg2+ and NAC–Ca2+) on water uptake, rate and duration of drug release, and PLGA degradation characteristics was extensively investigated. Drug distribution in the PLGA matrix as a function of NAC loading was studied by DSC, XRD, and SEM analysis.
MATERIALS AND METHODS
Materials
NAC was purchased from Sigma (St. Louis, MO, USA). N,N′ Diacetylcysteine (DAC) was obtained from Toronto research chemicals Inc. (North York, ON, Canada). PLGA 50:50 [I. V=0.57 dl/g and Mw=51 kDa, end-capped (end group=lauryl ester)] was purchased from (Medisorb, Alkermes, Cambridge, MA, USA). Potassium phosphate monobasic (KH2PO4), magnesium hydroxide (Mg (OH)2), sodium hydroxide (NaOH) and calcium hydroxide (Ca (OH)2), were obtained from Sigma-Aldrich Chemical (St. Louis, MO, USA). All other reagents and solvents were of analytical grade or purer and purchased from commercial suppliers.
Preparation of NAC Salts (NAC–Na+, NAC–Mg2+ and NAC–Ca2+)
NAC salt forms were prepared by simple titration of the NAC free acid with bases, NaOH, Mg(OH)2 or Ca(OH)2. Deionized water (10 ml) was purged with nitrogen gas for 3 h before addition of ∼750 mg NAC free acid. The NAC free acid solution was titrated with bases at the acid: base molar ratio of ∼2:1 (Mg(OH)2 and Ca(OH)2) and ∼1:1 (NaOH) slowly under constant stirring and the pH was monitored with pH electrode (Orion Research, Boston, USA). Titration was stopped at a final pH of the NAC–Mg(OH)2 or NAC–Ca(OH)2 or NAC–NaOH solution was 6.1. The resulting solutions were freeze-dried immediately and freeze-dried products were passed through a stainless steel sieve to obtain the particle size of <90 μm.
Preparation of NAC, NAC–Na+, NAC–Mg2+, and NAC–Ca2+/PLGA Cylindrical Implants
Solvent extrusion and fluid energy micronization (FEM) methods, as described previously (17), were used for the preparation of PLGA cylindrical implants. Briefly, the required amount of NAC [1 and 3.5% (w/w)] was dissolved in acetone. Then, PLGA 50:50 (i.v=0.57 dl/g) was dissolved in NAC-acetone solution to make a final polymer concentration of 55% (w/w) with respect to solvent weight. In case of millicylinder formulations to be loaded with 6.5, 8.0 and 10% (w/w) NAC with respect to polymer weight, the FEM technique was used in an attempt to reduce the drug particle size. This involved dissolving the NAC in excess acetone followed by evaporation of excess acetone to get polymer concentration of 55% (w/w) with respect to solvent before extrusion. NAC–Na+, NAC–Mg2+ and NAC–Ca2+/PLGA millicylinders were prepared by dispersing the required amount of NAC–Na+, NAC–Mg2+ or NAC–Ca2+ (sieved to <90 μm) in PLGA/acetone solution. For all formulations, drug-polymer solution or suspension was loaded into a 3-ml syringe and slowly extruded into the silicone tubing (I.D=0.8 mm) at approximately 0.1 ml/min. The solvent extruded solution/suspension was dried at room temperature for 48 h and then in a vacuum oven at 40°C for another 72 h. The silicone tubing was carefully cut to remove the cylindrical implants and only solid cylindrical implants were used in all the studies. Various millicylinder formulations compositions and their evaluation results are given in Table I.
Table I.
Evaluation of the Microencapsulation of NAC in PLGA Implants
| Millicylinder formulationsa | Theoretical loading (% w/w) | Actual loading (% w/w)b | Encapsulation efficiency (%)b | Tg (°C) |
|---|---|---|---|---|
| Blank | – | – | – | 25.2 |
| NAC free acid | 1.01 | 0.96±0.05 | 95.0±4.4 | 21.9 |
| 3.52 | 2.9±0.1 | 82.4±1.7 | 18.8 | |
| 6.49 | 6.1±0.3 | 93.6±4.1 | 27.3 | |
| 8.0 | 6.8±0.2 | 84.5±1.9 | 27.9 | |
| 9.99 | 8.5±0.1 | 87.9±1.2 | 29.0 | |
| NAC salts | ||||
| NAC–Mg2+ | 9.99 | 8.5±0.1 | 87.0±1.4 | 32.2 |
| NAC–Ca2+ | 10.0 | 9.7±0.6 | 98.4±5.9 | 34.5 |
| NAC–Na+ | 9.98 | 8.7±0.6 | 87.1±5.5 | N. Dc |
Millicylinders were prepared using PLGA 50:50 (i.v=0.57 dl/g)
Mean±S.E, n=3
Not determined
NAC HPLC Assay
All HPLC assays were performed on a Waters 2695 alliance system (Milford, MA, USA) consisting of a 2996 Photodiode array detector and a personal computer with Empower 2 Software. An Econosphere C18 (4.6×250 mm) reverse phase column (Alltech, USA) was used at a flow rate of 1.2 ml/min. The mobile phase was 0.05 M KH2PO4 and detection wavelength was set to 210 nm (15). Standard curves of NAC and DAC was established and concentration of unknown samples was calculated from their standard curves. The relative molar extinction coefficient of the two species (DAC/NAC) at the 210 nm was determined to be 0.98.
Determination of Drug Loading
NAC or NAC salts (NAC–Na+, NAC–Mg2+ and NAC–Ca2+) encapsulated PLGA implants were weighed and placed in 5 ml glass vials. To these vials, 1 ml of methylene chloride and 2 ml N2-purged 0.05 M KH2PO4 were added followed by spinning for 5 min. The aqueous layer containing NAC was diluted suitably using the N2-purged 0.05 M KH2PO4 and analyzed by HPLC. The extraction efficiency of NAC into 0.05 M KH2PO4 was determined by using known concentrations of NAC solution in a similar manner as with samples. A extraction efficiency of 95.9±4.5% (mean±SE, n=3) was determined. The NAC loading was calculated as the percentage of amount of NAC versus the total weight of mixture (i.e., NAC and PLGA).
Evaluation of NAC Release from PLGA Implants
In vitro release studies were conducted in N2-purged phosphate buffered saline (PBS, pH 7.4) under perfect sink conditions. About 5 mg NAC, NAC–Na+, NAC–Mg2+ and NAC–Ca2+-containing PLGA millicylinders were weighed and placed in 1-ml ampoules (Separate ampoules for each sampling interval). Exactly 1-ml PBS was added to all ampoules and sealed under vacuum in order remove the oxygen from the head-space. The ampoules were placed in an incubator maintained at 37°C and shaken at 100 RPM. At predetermined time intervals (1, 3, 7, 14, 21, and 28 days), three ampoules were taken out, broken, and release medium was suitably diluted and cumulative amount of released NAC was determined by HPLC.
Measurement of pH
The change in pH of the release medium was monitored over a period of 7-days when incubated with 0, 3.5, 6.5, and 10 wt% NAC and 10 wt% NAC–Mg2+ and NAC–Ca2+ salt-loaded PLGA millicylinders. The pH of the PBS was measured using pH meter (Orion Research, Boston, USA) against pH 4, 7 and 10 standards (Fisher Scientific, USA) at various time periods (1, 3, and 7 days).
Measurement of Water Uptake of PLGA Implants
PLGA millicylinders containing NAC or NAC salts were incubated in N2-purged PBS at 37°C in sealed ampoules as described for evaluation of NAC release from PLGA implants. At predetermined time intervals (1, 3, and 7 days), three ampoules were taken out and broken, millicylinders were blotted with tissue paper, weighed immediately, and then vacuum dried. The water content of millicylinders was calculated by
| (1) |
where W1 and W2 are the weights of the wet and dry millicylinders, respectively.
Mass Loss of PLGA Implants
PLGA millicylinders containing NAC or NAC salts were incubated in N2-purged PBS at 37°C in sealed ampoules as described for evaluation of NAC release from PLGA implants. At the end of each immersion (1, 3, and 7 days), millicylinders were removed and dried at room temperature in a vacuum oven to a constant weight. The percentage mass loss of each millicylinder (n=3) was calculated as shown:
| (2) |
Stability of NAC in PBS and in the Polymer
A stability study of NAC in PBS was carried out in the presence and absence of oxygen. In a typical reaction conditions, in each set of experiments, where PBS was exposed to atmospheric conditions for 48 h (this exposure time was sufficient to saturate PBS with oxygen). In another set of experiments, the PBS was degassed by purging with nitrogen gas for 3 h (this time was sufficient to remove complete oxygen) (18) followed by applying vacuum. In case of stability study with oxygen, required solutions of the NAC were prepared in oxygen saturated PBS and placed in 1 ml separate 1-ml plastic tubes. On the other hand, NAC solutions were prepared in N2-purged PBS, taken into separate 1-ml ampoules and sealed under vacuum. All the tubes and ampoules were placed in an incubator maintained at 37°C and shaken at 100 RPM. At predetermined time intervals, three tubes or ampoules were taken out and fraction of NAC remaining was determined by HPLC. To estimate the stability of NAC in the polymer, millicylinders containing 4.2 wt% NAC were incubated in oxygen containing PBS in 1-ml plastic tubes and N2-purged PBS in sealed ampoules at 37°C. After each time point, millicylinders were dissolved in methylene chloride and extracted with 2-ml N2-purged 0.05 M KH2PO4. The stable NAC was determined by HPLC as described above.
Determination of Glass Transition Temperatures
Glass transition temperatures (Tg) of all millicylinders were measured by a differential scanning calorimeter (TA Instruments, New Castle, DE, USA). Millicylinder samples (∼7 mg) were sealed in aluminum hermetic pans and thermograms were determined by first cooling the sample to −40°C, then heating to 70°C at a heating rate of 10°C/min under purged nitrogen atmosphere. The startpoint of the second run was used for Tg calculation.
X-ray Diffraction (XRD)
The physical state of NAC in the polymer was examined by measuring the XRD pattern of NAC free acid, blank and NAC/PLGA millicylinders using a Scintag powder X-ray diffractometer (Scintag, CA, USA). The X-ray source was copper Kα (40 kV, 30 mA), and the scanning speed was 2 deg/min.
Scanning Electron Microscopy
The surface morphology of NAC/PLGA and NAC salts/PLGA millicylinders was examined using a Hitachi S3200N scanning electron microscope (Hitachi, Tokyo, Japan). The millicylinders were fixed previously on a brass stub using double-sided adhesive tape and then were made electrically conductive by coating, in a vacuum, with a thin layer of gold (approximately 3 to 5 nm) for 60 s at 40 W. The cross-sectional and surface view images of millicylinders were taken at an excitation voltage of 12 and 20 kV.
Gel Permeation Chromatography
Weight-averaged molecular weight (MW) of the degraded PLGA was measured by gel permeation chromatography (GPC). The Waters 1525 GPC system (Waters, Milford, MA, USA) consisted of two Styragel columns (HR 1 and HR-5E columns, Waters, Milford, MA, USA) connected in series (7.8×300 mm each), a binary HPLC pump, waters 717 plus autosampler, Waters 2414 refractive index detector and Breeze™ software to compute molecular weight distribution. Sample solutions in tetrahydrofuran (THF) at a concentration of ∼4 mg/ml were filtered through a 0.2 μm hydrophobic fluoropore (PTFE) filter (Millipore, Bedford, USA) before injection into the GPC system and were eluted with THF at 1 ml/min. The weight average molecular weight (Mw) of each sample was calculated using monodisperse polystyrene standards, Mw 2,330–110,000 Da.
RESULTS AND DISCUSSION
Microencapsulation of NAC in PLGA Millicylinders
To develop injectable PLGA implants for slow release of NAC we selected the millicylinder configuration, similar to the Zoladex® (Goserelin acetate) implant (19), because of the simplicity of preparation, the ability to accurately control drug loading, and to minimize migration of the implant from the implant site following administration. PLGA millicylinders with 1–10 wt% of NAC (Table I) were prepared by solvent extrusion and FEM methods using acetone as a solvent (17). Under the polymer extrusion conditions only the 1 and 3.5% (w/w) drug-loaded millicylinders could be prepared with completely dissolved drug. In case of formulations with 6.5, 8.0 and 10% (w/w) drug load (theoretical), the FEM method was adopted. The scanning electron micrographs (SEM) of cross-sections of the resulting polymer matrices are displayed in Fig. 1. At low drug loading (e.g., 1 and 3.5 wt%) uniformly smooth cross-sections were observed with the absence of drug particles at the level of detection of the imaging. At higher drug loading (e.g., 6.5, 8, and 10 wt%), discrete drug particles uniformly dispersed throughout the polymer are clearly visible. As shown in Table I, in each formulation, the encapsulation efficiency was very high (82–98%), further supporting a good drug encapsulation.
Fig. 1.

Effect of NAC loading on inner morphology of PLGA 50:50 (i.v=0.57 dl/g) millicylinders. SEM images are displayed for 0 (a), 1 (b), 3.5 (c), 6.5 (d), 8 (e), and 10 (f) wt% NAC (theoretical) loaded millicylinders.
To further assess the polymer matrix and the level of miscibility of NAC in the polymer phase, both XRD and DSC analyses were employed. The XRD patterns of the same group of NAC/PLGA millicylinders are displayed in Fig. 2. As shown in Fig. 2a, NAC displayed several peaks corresponding to its crystalline form, whereas PLGA (Fig. 2b) was mainly amorphous with a poorly crystalline part, likely corresponding to crystalline oligomeric stereocomplex (20,21). The major diffraction peaks of NAC crystals were observed at the 2θ values of 18.4, 19.9, 21.0, 22.8, 24.1, 26.2, 26.7, 30.1 and 32.2°, among which the peak with higher intensity was observed at 2θ value of 26.7°. The diffraction peaks of NAC crystals were absent in the XRD pattern of millicylinders with 1 and 3.5% (w/w) NAC loadings (Fig. 2c and d) and only the XRD pattern characteristic of the pure polymer was observed. These results strongly suggested that when the NAC loading in the implant is less than 3.5% (w/w), NAC molecules dissolved in the polymer matrices. Millicylinders loaded with 6.5 and 10% (w/w) NAC showed major crystal peaks of NAC but with reduced intensity. Further, intensity of crystal peaks of NAC increased when the drug loading was increased from 3.5–10 wt% (Fig. 2e and f), suggesting that NAC crystals exist in implant as a dispersion in the polymer matrices together with NAC molecules dissolved in the matrices.
Fig. 2.

Effect of NAC loading on its miscibility with PLGA 50:50 (i.v=0.57 dl/g). X-ray diffraction patterns shown for NAC free acid (a) and 0 (b), 1 (c), 3.5 (d), 6.5 (e), and 10 (f) wt% NAC (theoretical) loaded millicylinders.
The DSC scans of NAC/PLGA millicylinders were consistent with XRD data. As seen in Table I, the Tg of the blank implant was reduced relative to that of the unprocessed polymer (Tg=45°C) owing to residual acetone in the polymer. Note that in the Zoladex® (Goserelin acetate) implant a specification of <2.5% acetic acid (19), also suggesting the difficulty of removing polar organic solvents from this polymer prepared on this length scale (∼1 mm diameter). As NAC was introduced into the polymer at low loading, plasticization was observed, the polymer Tg decreased to 21.9 and 18.8°C for 1 and 3.5 wt% loading, respectively. By contrast, in higher loading samples (6.5, 8, and 10 wt% NAC), where both NAC and PLGA phases were previously observed by SEM, slightly higher Tg (27.3–29°C) from placebo were observed, suggestive of improved acetone removal with increased pores formed by drug particles. Hence, both the plasticization of the low loading samples and the transition of the DSC data between 3.5 and 6.5 wt% NAC loaded samples are suggestive of moving from a monophasic to a biphasic system between 3.5 and 6.5 wt% drug loading.
In Vitro Drug Release Characteristics of NAC/PLGA Implants
Influence of Drug Loading on the Rate and Duration of NAC Release
To examine the controlled release behavior of NAC from the PLGA implants, millicylinders were placed in N2-purged PBS under perfect sink conditions and sealed in ampoules. Without O2-removal oxidation of the free thiol of NAC was rapid (see below). Both the release and polymer erosion kinetics were examined. In the course of release studies, it was observed that the rate and duration of NAC release from PLGA implants was uncharacteristically fast. Two different techniques were undertaken to decrease the release rate and improve the duration of NAC release from PLGA implant: the first was to vary the NAC loading (Table I), and the second was to employ two NAC salts (NAC–Mg2+ and NAC–Ca2+).
The influence of NAC loading on the rate and duration of drug release is shown in Fig. 3. As shown in Fig. 3a, it can be seen that the initial burst effect was strongly affected by the loading of NAC in the PLGA implant. As loading increased, in all cases release of NAC from PLGA implant rose steadily. For example, after 1-day of incubation, millicylinders loaded with 1, 3.5, 6.5, 8, and 10 wt% NAC exhibited 2, 5, 16, 20, and 34% drug release, respectively. Lowest initial burst effect was observed by millicylinders loaded with 1 and 3.5 wt% NAC. Further, after 7-days of incubation, 1, 3.5, 6.5, 8, and 10 wt% NAC loaded millicylinders exhibited 5.7, 7.8, 49.2, 61.2, and 77.1% drug release, respectively. To understand the mode of drug release within 7 days of incubation, the water uptake in the polymer was monitored and surface morphology of millicylinders after 7-days of incubation was observed by SEM. As the loading is increased, the water uptake by the polymer increased markedly (Fig. 4). For example, after 1-day of incubation, the water uptake by the polymer loaded with 1, 3.5, 6.5, 8, and 10 wt% NAC was respectively 10.9, 27.8, 52.7, 56.7, and 129.4% relative to initial polymer mass. NAC is a small molecule (MW=163.2 g) with high water solubility (∼100 mg/ml) giving rise to a strong osmotic pressure change in the polymeric matrix. After 7-days of incubation, the surface of implants loaded with 1 and 3.5 wt% NAC (Fig. 5b and c) were non-porous, whereas, the implants loaded with 8 and 10 wt% NAC were more porous (Fig. 5d and e). It is likely that molecular drug dispersion in the polymer phase, lower water uptake, non-porous polymeric matrices observed with 1 and 3.5 wt% drug-loaded formulations resulted in a very low initial burst effect and slower drug diffusion through the polymer phase during the 7-days release period. By contrast, more pronounced burst effect exhibited by millicylinders loaded with 6.5, 8 and 10 wt% drug-load can be attributed to higher water uptake and porous polymeric matrices formed after dissolution of drug crystals.
Fig. 3.
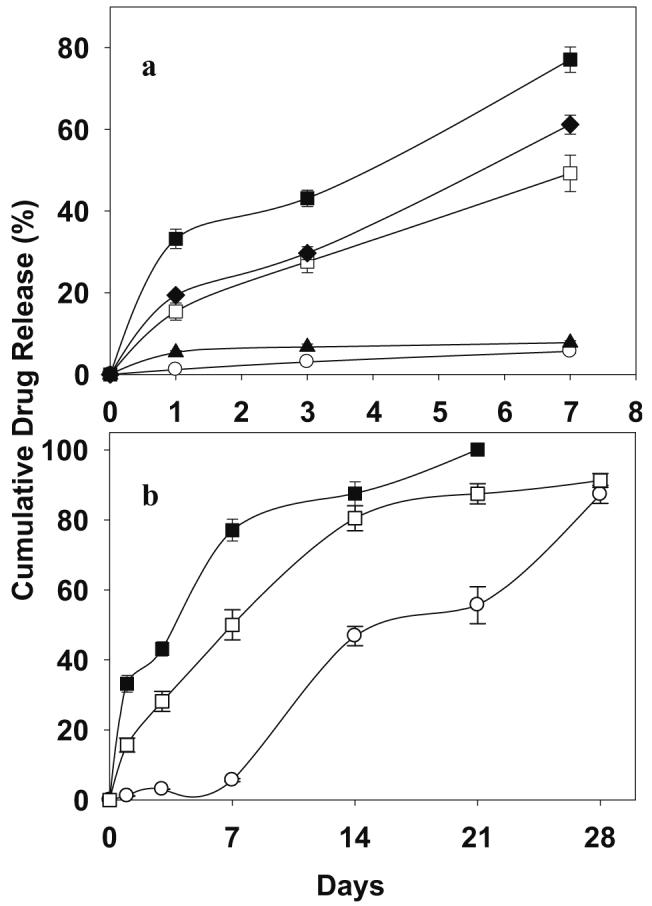
Effect of NAC loading on initial burst effect (a) and duration of drug release (b) characteristics of PLGA 50:50 (i.v=0.57 dl/g) millicylinders. Drug loadings were 1 (open circle), 3.5 (filled triangle), 6.5 (open square), 8 (filled diamond), and 10 (filled square) wt% NAC (theoretical). Studies were carried out in N2-purged PBS at 37°C and symbols represent mean±SE, n=3.
Fig. 4.

Effect of NAC loading on water uptake in the PLGA 50:50 (i.v=0.57 dl/g) millicylinder. Formulations were blank (open triangle) and 1 (open circle), 3.5 (filled triangle), 6.5 (open square), 8 (filled diamond), and 10 (filled square) wt% NAC (theoretical) loaded millicylinders. Studies were carried out in N2-purged PBS at 37°C and symbols represent mean±SE, n=3.
Fig. 5.

Effect of NAC loading on surface morphology of PLGA 50:50 (i.v=0.57 dl/g) millicylinders after 7-days of immersion in N2-purged PBS at 37°C. SEM images are displayed for 0 (a), 1 (b), 3.5 (c), 8 (d) and 10 (e) wt% NAC (theoretical) loaded millicylinders.
On the other hand, influence of NAC loading on the drug release duration was studied using PLGA millicylinders loaded with 1, 6.5 and 10 wt% NAC (Fig. 3b). Again, NAC content exhibited a considerable effect on the duration of drug release. For instance, the release profile from millicylinders loaded with 1 wt% NAC was multiphasic, i.e., lag phase, first burst, slow release and then a second burst (Fig. 3b). In the lag phase, only 5.6% drug was released after the 7 days of immersion. By contrast, millicylinders loaded with 10 wt% NAC exhibited shorter release duration. About ∼77% drug released after 7 days and complete drug release occurred within 21 days, owing to high polymer porosity observed at this loading. By contrast, millicylinders with 6.5 wt% drug-load exhibited 91% drug release slowly and continuously over 28 days. Millicylinders with low drug load and optimum porosity in the polymer exhibited longer duration of drug release. It can be emphasized that plasticization of the polymer, increased water uptake, and polymer porosity and enhanced polymer degradation (see below) observed by NAC/PLGA implants led to high burst effect and short release duration.
NAC Increases Rate of PLGA Degradation
Understanding the effect of incorporated compounds on polymer degradation is of particular importance in drug delivery systems. The influence of NAC free acid on the polymer degradation (7-days) in N2-purged PBS at 37°C was studied by monitoring the polymer Mw decline, change in pH of the release medium and mass loss (Fig. 6). Blank millicylinders and PLGA millicylinders with 3.5, 6.5, and 10 wt% drug-load were selected for degradation study. As shown in the Fig. 6a, the degradation rate of PLGA is enhanced significantly by NAC free acid and affected slightly by NAC loading, indicating the poor suitability of NAC/PLGA implants for prolonged drug release. The initial Mw of the polymer for 3.5, 6.5 and 10 wt% drug loaded formulations was 48.4, 53.0 and 49.8 kDa, respectively. After 1-day of incubation, the Mw of 3.5, 6.5 and 10 wt% drug loaded polymer decreased to 8.2, 10.9 and 9.7 kDa, respectively. As previously shown, the presence of NAC free acid in PLGA resulted in plasticization of the polymer (see Table I for Tg values) and higher water uptake in the polymer (Fig. 4). Thus, polymer degradation takes place throughout the device upon exposure to aqueous media (“bulk erosion”) (22). It is likely that increased number of carboxylic acid end groups generated by polymer degradation and free carboxylic acid groups present in NAC molecules led to acceleration in polymer degradation (general acid catalysis) and increased solid-state diffusion of the drug (23). However, we cannot rule out an additional contribution of the free thiol group to accelerate PLGA hydrolysis.
Fig. 6.

Effect of NAC loading on PLGA 50:50 (i.v=0.57 dl/g) degradation. NAC/PLGA implants were incubated in N2-purged PBS at 37°C. Weight average molecular weight (Mw) (a), release medium pH (b), and mass loss (c) profiles are shown for 0 (filled circle), 3.5 (open circle), 6.5 (filled inverted triangle), and 10 (open triangle) wt% NAC (theoretical) loaded millicylinders. Symbols represent mean±SE, n=3.
The mass loss of NAC/PLGA millicylinders was also not surprisingly higher than that of NAC-free millicylinder (Fig. 6c), suggesting again that NAC increased the polymer degradation. However, the polymer mass loss increased as the drug loading is increased. For example, after 7-days of incubation, the mass loss for 3.5, 6.5, and 10 wt% NAC loaded implants was 3.9, 6.7, and 9.7%, respectively. It is apparent that mass loss of the PLGA implant increased as the polymeric matrix became porous during the 7-days incubation, suggesting considerable amount of water-soluble oligomers/monomers diffusion into the surrounding medium. The decline in pH of the release medium supported this phenomenon (Fig. 6b). It should be emphasized that the effects of NAC free acid on PLGA degradation characteristics are complex and depend on numerous parameters including acid catalysis induced by NAC free acid and oligomers/monomers, neutralization of carboxylic end groups, porosity, loading and physical state of NAC.
Chemical Stability of NAC
In O2-saturated solutions, solvated electron give rise to a highly reactive superoxide radical () (24). The superoxide radical reacts with the free thiol of NAC and forms cystine (DAC) (24-26). The mechanism of formation of cystine (DAC) and by-products (Eq. 3) is proposed by Benrahmoune et al. (26). Therefore, we examined the stability of NAC in solution (PBS, pH 7.4) and in the polymer (Fig. 7). The degradation (oxidation) product of NAC was confirmed by comparing the chromatographic mobilities of NAC and DAC. The retention time of the oxidation product of NAC in the experimental conditions was same as that of standard sample of DAC. It was demonstrated that the DAC is the only degradation product of NAC in the PBS (pH 7.4) and polymer at 37°C. As shown in Fig. 7a, this is evident from the molar equivalent decrease in NAC concentration and increase in DAC concentration as a function of time. This observation made is consistent with the reaction scheme shown in Eq. 3
| (3) |
Fig. 7.

Chemical stability of N-acetylcysteine (NAC). (a) Fraction of NAC remaining (filled circle) or N, N′-Diacetylcysteine (DAC) formed (filled square) in O2-saturated PBS; (b) Fraction of NAC remaining in O2-saturated PBS (filled square) and N2-purged PBS (filled circle); (c) Fraction of NAC remaining in the PLGA 50:50 (i.v=0.57 dl/g) polymer over a period of 7 days when incubated in O2-saturated PBS (filled circle) and N2-purged PBS (open circle). Concentration of NAC in (a) and (b) was 12.25 μM. NAC in the polymer (c) was 4.2 wt%. Studies were carried out at 37°C, symbols represent mean±SE (n=3).
Importantly, the oxidation of NAC could be prevented by removing the oxygen from the PBS (Fig. 7b). More than 98% NAC was stable in N2-purged PBS after 48 h of incubation. Furthermore, the stability of NAC in the polymer incubated with the O2-saturated and N2-purged PBS is shown in Fig. 7c. After 7-days, 68 and 90% NAC was stable in the polymer incubated with O2-saturated and N2-purged PBS, respectively, suggesting the potential for NAC stability in the polymer if the oxygen were removed from the release medium. As oxygen will be present at the tissue sites for NAC release, we are currently evaluating the stability of the NAC free thiol group in vivo. Should in vivo stability become problematic, we will explore strategies such as introduction of reducing equivalents like NADH into the implant, to maintain the NAC thiol group in its reduced state. In vivo stability kinetics of NAC will be reported in a subsequent manuscript, and was beyond current scope.
Improved Release Performance of NAC from PLGA Implants by Conversion of NAC Free Acid to NAC–Mg2+ and NAC–Ca2+ Salts
Our initial findings described above point to poor suitability of NAC/PLGA millicylinders for prolonged drug release applications: the partitioning of the NAC free acid in PLGA gives rise to high initial burst, fast release duration and enhanced degradation of the polymer. It is interesting to note that a high level of water-soluble acid i.e. impurities is known to cause depolymerization of PLGA on storage (27). To obviate these issues, we formed the NAC–Mg2+ and NAC–Ca2+ salts by simple titration of the NAC free acid with the hydroxides of the divalent cations (Mg(OH)2 and Ca(OH)2). NAC–Mg2+ and NAC–Ca2+ salts were also selected instead of NAC–Na+ salt to decrease the solubility, and to minimize the initial burst (see below) of the strongly osmotic drug molecule. About 99% NAC was recovered from its salts, suggesting that oxidation of NAC was successfully prevented during the acid–base reaction. Further, the conversion of NAC free acid to NAC–Mg2+ and NAC–Ca2+was confirmed by FTIR (data not shown), where the absence of O–H stretching mode (voh) of carboxylic acid (NAC–COOH) was observed in the FTIR spectra of the NAC salts. The absorption peak due to free thiol (SH) stretching band (vsh) at 2545 cm−1was observed in the FTIR spectra of NAC. NAC–Mg2+ and NAC–Ca2+ salts showed absorption peaks at 2,547 and 2,549 cm−1, respectively suggesting that free thiol was not involved in complexation. The absence of formation of complex between carboxylate function and the thiol of NAC (28) has been carefully verified experimentally and selective complex formation of divalent cations with NAC's carboxylate ions has been demonstrated (29). The NAC salts were encapsulated in PLGA implant by solvent-extrusion method with an encapsulation efficiency of over 87%. Uniform distribution of discrete salt particles throughout the polymer matrix can be observed in Fig. 8.
Fig. 8.

Inner morphology of 10 wt% (theoretical) NAC–Mg2+ (a) and NAC–Ca2+ (b) salts loaded PLGA 50:50 (i.v=0.57 dl/g) millicylinders.
Decreased Burst Effect and Prolonged Release Duration of NAC by NAC–Mg2+ and NAC–Ca2+ salt/PLGA Implants
The influence of encapsulation of NAC salts (NAC–Na+, NAC–Mg2+ and NAC–Ca2+) in PLGA on the initial burst effect and duration of drug release is shown in Fig. 9. As shown in Fig. 9a, NAC–Mg2+ and NAC–Ca2+ salts were found to be more effective in decreasing the initial burst effect when compared to the NAC–Na+. NAC–Na+/PLGA millicylinders exhibited a very high initial burst effect (∼53% release after 1-day) and short release duration (∼94% release after 7-days). In contrast, NAC–Ca–2+/PLGA millicylinder exhibited the least initial burst effect among all the 10 wt% loaded formulations studied. For example, after 1-day of incubation, NAC free acid/PLGA, NAC–Mg2+/PLGA and NAC–Ca–2+ millicylinders exhibited ∼34, 22 and 13% drug release, respectively. The water uptake by the polymer containing NAC–Na+ was also much higher than those containing NAC free acid, NAC–Mg2+ and NAC–Ca2+ (Fig. 10). On the other hand, the water uptake in the polymer was also affected by the type of divalent cationic salts (Mg2+– or Ca2+–) encapsulated. For example, the NAC–Mg2+/PLGA millicylinders exhibited higher water uptake than NAC–Ca2+/PLGA. Similarly, water uptake in the polymer corresponded with higher release rate of NAC from NAC salt/PLGA implants. Additional investigations entailed SEM morphologic assessment of NAC salts/PLGA millicylinders after 7-days of release (Fig. 11). These results were consistent with the profiles of water uptake in the polymer and release, and confirmed that polymeric matrices loaded with NAC–Na+ and NAC–Mg2+ were more porous than NAC–Ca2+/PLGA matrix. Since the NAC–Na+/PLGA implant exhibited high burst and short release duration, the NAC–Mg2+ and NAC–Ca2+/PLGA implants were considered lead formulations and used for additional studies.
Fig. 9.

Effect of drug form on initial burst effect (a) and duration of drug release (b) characteristics of PLGA 50:50 (i.v=0.57 dl/g) millicylinders. Millicylinders were loaded with 10 wt% (theoretical) NAC free acid (filled circle), NAC–Mg2+ (open circle), NAC–Ca2+ (filled inverted triangle), and NAC–Na+ (filled square) salts. Studies were carried out in N2-purged PBS at 37°C, symbols represent mean±SE, n=3.
Fig. 10.

Effect of drug form on water uptake in the PLGA 50:50 (i.v=0.57 dl/g) millicylinder. Millicylinders were loaded with 10 wt% (theoretical) NAC free acid (filled circle), NAC–Mg2+ (open circle), NAC–Ca2+ (filled inverted triangle), and NAC–Na+ (filled square) salts. Studies were carried out in N2-purged PBS at 37°C, symbols represent mean±SE, n=3.
Fig. 11.

Surface morphology of 10 wt% (theoretical) NAC–Mg2+ (a), NAC–Ca2+ (b) and NAC–Na+ (c) salts loaded PLGA 50:50 (i.v=0.57 dl/g) millicylinders after 7 days of immersion in N2-purged PBS at 37°C.
The cumulative drug release profiles of NAC–Mg2+ and NAC–Ca2+ salts/PLGA millicylinders relative to the NAC free acid/PLGA millicylinder control over a period of one month is displayed in Fig. 9b. Formation of NAC–Mg2+ and NAC–Ca2+ salts not only decreased the burst effect but also prolonged the drug release duration when compared to NAC free acid itself. However, NAC–Ca2+ was found to be more effective in sustaining the NAC release from PLGA implants. For example, after 21 days of incubation, NAC–Mg2+/PLGA and NAC–Ca2+/PLGA millicylinders exhibited 95 and 69% drug release, respectively. It is noteworthy to mention that, even after 28-days of release study, more than 80% of NAC was stable in the release medium.
Conversion of NAC Free Acid to NAC–Mg2+ and NAC–Ca2+ Salts Inhibit NAC-Catalyzed PLGA Degradation
The variation in Mw, polymer mass loss, and release medium pH for PLGA millicylinders containing NAC–Mg2+ and NAC–Ca2+ salts was compared with NAC/PLGA millicylinders in Fig. 12a,b, and c, respectively. NAC catalyzed PLGA degradation was markedly inhibited by encapsulating the salts. For instance, after 7-days of incubation, for 10 wt% NAC free acid encapsulated millicylinder, the Mw of PLGA was reduced by ∼85%. Whereas, for PLGA encapsulated with NAC–Mg2+ and NAC–Ca2+ salts, the rate of reduction in Mw was slow over a period of 1-week. After 7-days, the reduction in Mw was about 15 and 20% for PLGA containing NAC–Mg2+ and NAC–Ca2+ salts, respectively. As shown in Fig. 12b, the mass loss of NAC–Mg2+ and NAC–Ca2+ salt-loaded PLGA millicylinders was lower than that of the NAC free acid control. Consistent with these results, the release medium pH remained greater for the 10 wt% NAC–Mg2+ and NAC–Ca2+ salts/PLGA millicylinders relative to the 10 wt% NAC free acid/PLGA millicylinder (Fig. 12c). In addition, conversion of NAC free acid to NAC–Mg2+ and NAC–Ca2+ salts inhibited the NAC free acid induced polymer plasticization (see Table I). The presence of base NAC–COO− vs. NAC–COOH obviously decreased the number of −COOH groups available for acid catalyzed degradation. Also, complexation of divalent ions to polyesters by ion–dipole interaction hypothesized to decrease the reactivity of ester linkage towards hydrolysis (30,31).
Fig. 12.

Effect of formation of NAC form on PLGA 50:50 (i.v=0.57 dl/g) degradation. Millicylinders were incubated in N2-purged PBS at 37°C over a period of 7 days. Weight average molecular weight (Mw) (a), mass loss (b), and release medium pH (c) profiles are shown for 10 wt% (theoretical) NAC free acid (filled circle), NAC–Mg2+ (open circle), and NAC–Ca2+ (filled inverted triangle) salts loaded millicylinders. Symbols represent mean±SE, n=3.
CONCLUSION
Encapsulation of NAC free acid in PLGA polymer resulted in plasticization, higher water uptake in the polymer and enhanced polymer degradation, resulting in higher burst effect and shorter release duration. The key issues associated with NAC free acid/PLGA cylindrical implants were overcome by conversion of NAC free acid to NAC–Mg2+ and NAC–Ca2+ salts. In NAC–Mg2+ and NAC–Ca2+/PLGA implants, the high initial burst, short release duration, and the general acid catalysis caused by the drug were each prevented and prolonged drug release could be achieved for over a month. The NAC–Mg2+ and NAC–Ca2+/PLGA implants described here represent promising formulations for local, site-specific controlled NAC release for chemopreventive applications.
ACKNOWLEDGEMENT
This study was supported by NIH R01 CA95901 (Mallery) and R01 HL68345 (Schwendeman). We thank Dr. Naír Rodríguez-Hornedo and A. Jayasankar for the technical assistance with the DSC measurements.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu JQ, Thun MJ. Cancer statistics. CA-Cancer. J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA. Head and neck cancer: Overview of recent developments and future directions. Semin. Oncol. 2000;27:1–4. [PubMed] [Google Scholar]
- 3.Ganly I, Kaye SB. Recurrent squamous-cell carcinoma of the head and neck: Overview of current therapy and future prospects. Ann. Oncol. 2000;11:11–16. doi: 10.1023/a:1008330026617. [DOI] [PubMed] [Google Scholar]
- 4.Shah NH, Railkar AS, Chen FC, Tarantino R, Kumar S, Murjani M, Palmer D, Infeld MH, Malick AW. A biodegradable injectable implant for delivering micromolecules and macromolecules using poly(lactic-co-glycolic) acid (PLGA) copolymers. J. Controlled Release. 1993;27:139–147. [Google Scholar]
- 5.Sartor O, Dineen MK, Perez-Marreno R, Chu FM, Carron GJ, Tyler RC. An eight-month clinical study of LA-2575 30.0 mg: A new 4-month, subcutaneous delivery system for leuprolide acetate in the treatment of prostate cancer. Urology. 2003;62:319–323. doi: 10.1016/s0090-4295(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 6.Sampath P, Brem H. Implantable slow-release chemotherapeutic polymers for the treatment of malignant brain tumors. Cancer Contl. 1997;5:130–137. doi: 10.1177/107327489800500204. [DOI] [PubMed] [Google Scholar]
- 7.Mallery SR, Pei P, Kang JC, Ness GM, Ortiz R, Touhalisky JE, Schwendeman SP. Controlled-release of doxorubicin from poly(lactide-co-glycolide) microspheres significantly enhances cytotoxicity against cultured AIDS-related kaposi's sarcoma cells. Anticancer. Res. 2000;20:2817–2825. [PubMed] [Google Scholar]
- 8.Crawford ED, Sartor O, Chu F, Perez R, Karlin G, Garrett JS. A 12-month clinical study of LA-2585 (45.0 MG): A new 6-month subcutaneous delivery system for Leuprolide acetate for the treatment of prostate cancer. J. Urology. 2006;175:533–536. doi: 10.1016/S0022-5347(05)00161-8. [DOI] [PubMed] [Google Scholar]
- 9.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in turner invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 10.Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 1999;189:300–308. doi: 10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Kurahara S, Shinohara M, Ikebe T, Nakamura S, Beppu M, Hiraki A, Takeuchi H, Shirasuna K. Expression of MMPs, MT–MMP, and TIMPs in squamous cell carcinoma of the oral cavity: Correlations with tumor invasion and metastasis. Head. Neck—J. Sci. Spec. 1999;21:627–638. doi: 10.1002/(sici)1097-0347(199910)21:7<627::aid-hed7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Garbett EA, Reed MWR, Brown NJ. Proteolysis in human breast and colorectal cancer. Brit. J. Cancer. 1999;81:287–293. doi: 10.1038/sj.bjc.6690689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franchi A, Santucci M, Masini E, Sardi I, Paglierani M, Gallo O. Expressionof matrix metalloproteinase 1, matrix metalloproteinase 2, and matrix metalloproteinase 9 in carcinoma of the head and neck—Correlation with p53 status, inducible nitric oxide synthase activity, and anigogenesis. Cancer Res. 2002;95:1902–1910. doi: 10.1002/cncr.10916. [DOI] [PubMed] [Google Scholar]
- 14.Pei P, Horan MP, Hille R, Hemann CF, Schwendeman SP, Mallery SR. Reduced nonprotein thiols inhibit activation and function of MMP-9: Implications for chemoprevention. Free Radical. Bio. Med. 2006;41:1315–1324. doi: 10.1016/j.freeradbiomed.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieutord A, Arnaud P, Dauphin JF, Brion F. Stability and compatibility of an aerosol mixture including N-acetylcysteine, netilmicin and betamethasone. Int. J. Pharm. 1999;190:103–107. doi: 10.1016/s0378-5173(99)00280-x. [DOI] [PubMed] [Google Scholar]
- 16.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life. Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou TH, Lewis H, Foster RE, Schwendeman SP. Development of a multiple-drug delivery implant for intraocular management of proliferative vitreoretinopathy. J. Controlled Release. 1998;55:281–295. doi: 10.1016/s0168-3659(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 18.Bleau G, Giasson C, Brunette I. Measurement of hydrogen peroxide in biological samples containing high levels of ascorbic acid. Anal. Biochem. 1998;263:13–17. doi: 10.1006/abio.1998.2801. [DOI] [PubMed] [Google Scholar]
- 19. http://www.accessdata.fda.gov/scripts/cder/onctools/labels.cfm?GN=goserelin%20acetate.
- 20.Seo SA, Choi HS, Khang G, Rhee JM, Lee HB. A local delivery system for fentanyl based on biodegradable poly(l-lactide-co-glycolide) oligomer. Int. J. Pharm. 2002;239:93–101. doi: 10.1016/s0378-5173(02)00074-1. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Vert M. Crystalline oligomeric stereocomplex as an intermediate compound in racemic poly(dl-lactic acid) degradation. Polym. Int. 1994;33:37–41. [Google Scholar]
- 22.Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)] Polym. Int. 2005;54:36–46. [Google Scholar]
- 23.Kang JC, Schwendeman SP. Determination of diffusion coefficient of a small hydrophobic probe in poly(lactide-co-glycolide) microparticles by laser scanning confocal microscopy. Macromolecules. 2003;36:1324–1330. [Google Scholar]
- 24.Benrahmoune M, Ghassah M, Abedinzadeh Z. Superoxide radicals action on N-acetylcysteine. J. Chim. Phys. PCB. 1997;94:257–261. [Google Scholar]
- 25.Bagiyan GA, Koroleva IK, Soroka NV, Ufimtsev AV. Oxidation of thiol compounds by molecular oxygen in aqueous solutions. Russ. Chem. B+ 2003;52:1135–1141. [Google Scholar]
- 26.Benrahmoune M, Therond P, Abedinzadeh Z. The reaction of superoxide radical with N-acetylcysteine. Free Radical. Bio. Med. 2000;29:775–782. doi: 10.1016/s0891-5849(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto HOM, Ogawa Y, Miyagawa T. US Patent 4,728,721 1988
- 28.Inczedy J, Marothy J. Metal-Complexes of N–Acetyl–Cysteine. Acta. Chim. Hung. 1975;86:1–2. [Google Scholar]
- 29.Smuleac V, Butterfield DA, Sikdar SK, Varma RS, Bhattacharyya D. Polythiol-functionalized alumina membranes for mercury capture. J. Membrane Sci. 2005;251:169–178. [Google Scholar]
- 30.Ara M, Watanabe M, Imai Y. Effect of blending calcium compounds on hydrolytic degradation of poly(dl-lactic acid-co-glycolic acid) Biomaterials. 2002;23:2479–2483. doi: 10.1016/s0142-9612(01)00382-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Zale S, Sawyer L, Bernstein H. Effects of metal salts on poly(dl-lactide-co-glycolide) polymer hydrolysis. J. Biomed. Mater. Res. 1997;34:531–538. doi: 10.1002/(sici)1097-4636(19970315)34:4<531::aid-jbm13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]


