Abstract
The first oral direct renin inhibitor, aliskiren, recently received approval for the treatment of hypertension. This article addresses the premise, promise, and potential limitations of this new class of renin-angiotensin system inhibitor. While aliskiren adds to a list of > 100 drugs approved for the treatment of hypertension, its introduction into clinical medicine is of particular interest because of the novel mechanism of action— inhibition of renin’s catalytic activity, the most proximal and rate-limiting step in renin-angiotensin system activation. By producing more complete renin-angiotensin system inhibition than with existing agents, direct renin inhibitors may afford greater protection from hypertensive complications. Other potential advantages include additional blood pressure reduction when used in combination therapy, a placebo-like side-effect profile, avid renal concentration, and long duration of action. Potential limitations include modest levels of blood pressure reduction that are equivalent to but not greater than angiotensin receptor blockers, reduced gastrointestinal absorption with a high fat meal, and large reactive increases in renin secretion—the functional importance of which is under intense investigation. The results of outcomes trials are eagerly awaited.
Keywords: renin, angiotensin, hypertension, aliskiren
Aliskiren, the first oral direct renin inhibitor, received Food and Drug Administration approval for the treatment of hypertension in March 2007. As direct renin inhibitors constitute the first new class of antihypertensive drug introduced in over a decade, aliskiren’s development and pharmacology have been reviewed in detail.1–3 In addition, most studies examining the drug’s effect on blood pressure and plasma renin have been extensively reviewed and critically evaluated.4 In contrast, this article addresses the premise, promise, and potential limitations of the new type of renin-angiotensin system inhibitor for hypertension.
WHY DO WE NEED ANOTHER RENIN-ANGIOTENSIN SYSTEM INHIBITOR?
The renin-angiotensin system is one of the most important pharmacologic targets for hypertension. Angiotensin converting enzyme inhibitors (ACE Inhibitors) and angiotensin receptor blockers (ARBs) are moderately effective blood pressure-lowering agents that prevent hypertensive complications with minimal side-effects.5 Aldosterone receptor blockers are effective adjunctive therapy for difficult hypertension of diverse etiology.6 So why do we need another class of renin-angiotensin system inhibitor in hypertension?
A large body of basic research suggests that renin-angiotensin system inhibitors should exert cytoprotective effects in target tissues, providing greater end-organ protection than other classes of antihypertensive agents that yield comparable reductions in blood pressure. However, clinical trials testing for ancillary blood pressure-independent benefits of renin-angiotensin system inhibitors have yielded mixed results—in part because ACE Inhibitors and ARBs do not produce complete renin-angiotensin system inhibition. By achieving more complete renin-angiotensin system inhibition,7 direct renin inhibitors may afford greater protection from hypertensive complications. The outcomes of clinical trials are eagerly awaited.
THE RENIN-ANGIOTENSIN SYSTEM IN HYPERTENSION AND CARDIOVASCULAR DISEASE
The renin-angiotensin system constitutes a short-term defense mechanism against hypovolemic hypotension (Figure 1). For example, when blood pressure falls due to dietary sodium restriction, renin is secreted from the juxtaglomerular cells, the sole site of renin synthesis and release. The enzymatic cleavage of angiotensinogen by renin is the rate-limiting step in the formation of angiotensin II, which elicits angiotensin II type 1 receptor-mediated vasoconstriction and aldosterone release causing renal sodium retention. These compensatory adjustments restore blood volume and blood pressure, which in turn cause feedback suppression of renin secretion. Additionally, angiotensin II itself causes feedback inhibition of renin secretion by acting on angiotensin II type 1 and angiotensin II type 2 receptors on the juxtaglomerular cells.8
Figure 1.
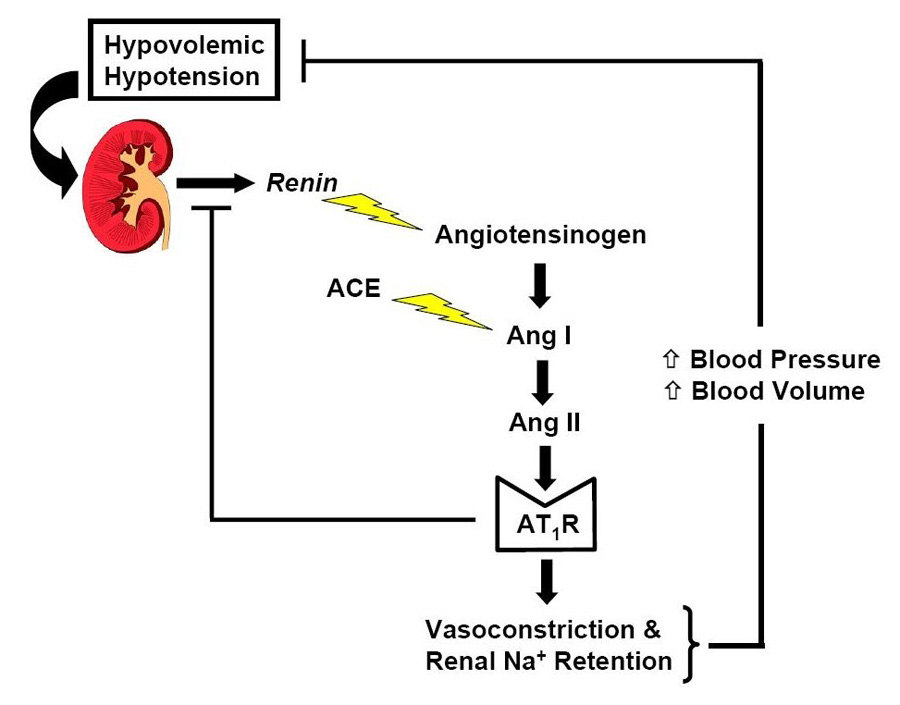
Appropriate activation of the renin-angiotensin system in the defense against hypovolemic hypotension.
ACE, angiotensin converting enzyme; Ang I, angiotensin I; Ang II, angiotensin II; AT1R, angiotensin II type 1 receptor; Na+, Sodium.
When dietary sodium is plentiful, the renin-angiotensin system should be continually suppressed and any degree of activation is inappropriate. In numerous rodent models of hypertension and atherosclerosis, inappropriate persistent angiotensin II type 1 receptor stimulation in target tissues drives downstream intracellular signaling pathways that culminate in the production of reactive oxygen species, endothelial dysfunction, vessel inflammation, cellular hypertrophy, and tissue fibrosis, as well as the release of aldosterone and norepinephrine, which further fuel the vasculopathy (Figure 2).9 Thus, augmented angiotensin II type 1 receptor-mediated signaling provides an attractive mechanistic explanation for the frequent coexistence of elevated blood pressure with insulin resistance and atherosclerosis in patients. The excessive renin-angiotensin system activation not only elevates blood pressure but also can accelerate detrimental effects of the excessive hemodynamic load on target tissues expressing angiotensin II type 1 receptors. As these detrimental effects can be abrogated by renin-angiotensin system inhibition—at least in rodents—angiotensin II type 1 receptor-signaling constitutes a major therapeutic target for interrupting every step in cardiovascular disease progression from vascular remodeling and atherosclerotic plaque progression to myocardial infarction, stroke, and death.10–17
Figure 2.
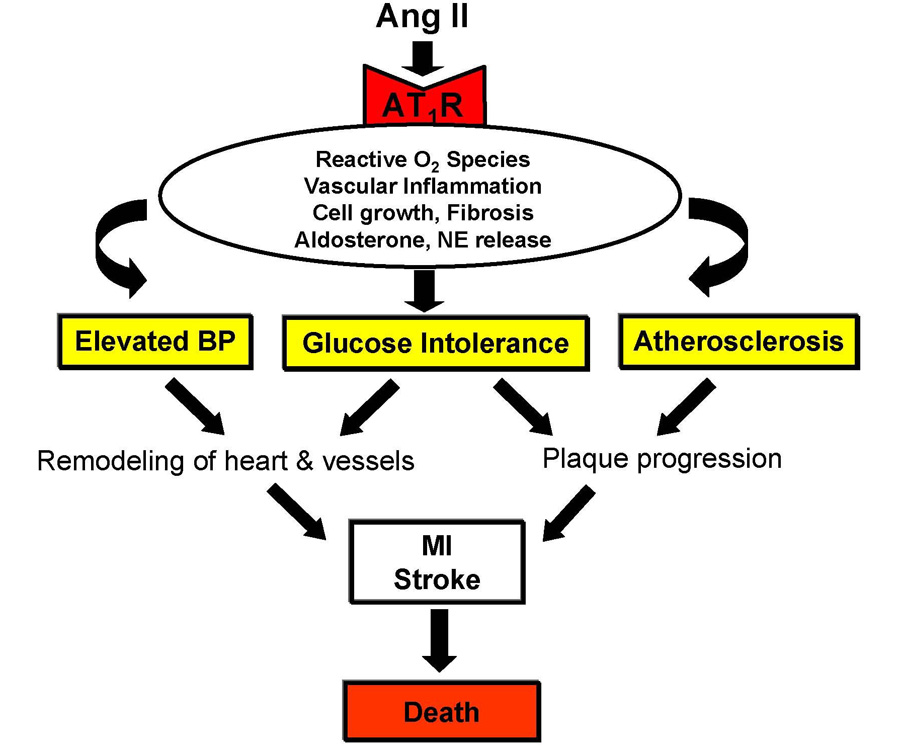
Central mechanistic role of angiotensin II type I receptor-mediated signaling in hypertension and cardiovascular disease progression.
NE, norepinephrine; BP, blood pressure; MI, myocardial infarction.
Despite a wealth of data from hypertension treatment trials, there still are large gaps in our understanding of the extent to which these basic science concepts can be translated to the clinical setting.
RENIN-ANGIOTENSIN SYSTEM INHIBITION FOR GREATER CARDIOVASCULAR PROTECTION?
For patients with left ventricular systolic dysfunction and heart failure, the mortality benefit of ACE Inhibitors and ARBs is unequivocal.18–23 However, for the majority of hypertensive patients with preserved left ventricular systolic function, there has been considerable debate as to whether renin-angiotensin system inhibitors afford greater cardiovascular protection than do other classes of antihypertensives.24, 25 Clinical trials have not settled the issue for several possible reasons including unequal blood pressure reductions between randomized groups and inadequate dosing with incomplete renin-angiotensin system blockade.
However, there is growing consensus among experts that reduction in blood pressure (i.e., hemodynamic load) explains most—but perhaps not all—of the cardiovascular benefit of antihypertensive therapy, regardless of drug class.5, 26–28 A recent large meta-regression analysis suggests that ACE Inhibitors can exert a small blood pressure-independent effect against myocardial infarction but not stroke, whereas calcium-channel-blockers exert a small blood pressure-independent effect against stroke but not myocardial infarction.26 Clearly, we would prefer a single strategy that afforded our patients optimal protection against both complications.
With their introduction in the 1990s, ARBs held exciting promise to offer greater cardiovascular protection than ACE Inhibitors by eliminating “ACE Inhibitor escape” and promoting activation of angiotensin II type 2 receptors which increase nitric oxide production and engage other cardioprotective mechanisms.29 However, in hypertension outcomes trials ARBs have not performed as well as initially expected, with some experts interpreting the data to suggest that ARBs might even precipitate myocardial infarctions—the so-called “myocardial infarction paradox.” 25 However, several recent sets of analyses indicate that ARBs reduce myocardial infarction risk solely by virtue of their ability to lower blood pressure, which is modest particularly at low doses.28, 30–32 The ARBs do not seem to possess any blood pressure-independent effects—either positive or negative—on myocardial infarction or stroke prevention.28, 29
RENIN-ANGIOTENSIN SYSTEM INHIBITION FOR GREATER RENAL PROTECTION?
A stronger case can be made that ACE Inhibitors and ARBs are superior to other antihypertensives for improving renal outcomes. The superiority of ACE Inhibitors over non-renin-angiotensin system inhibiting agents has been demonstrated repeatedly for slowing progression of hypertensive non-diabetic chronic kidney disease33, 34 and for slowing progression to renal failure in patients with type I diabetes.35 The superiority of ARBs has been demonstrated for slowing progression of nephropathy in patients with hypertension and type 2 diabetes.36–38 While ACE Inhibitors and ARBs clearly slow progression of proteinuric chronic kidney disease, they do not seem to prevent end-stage renal disease but rather delay its onset by ~ 6–8 months longer than other antihypertensive regimens.37
Recently, combined therapy with an ACE Inhibitor plus an ARB—for greater renin-angiotensin system blockade—showed greater reduction in proteinuria and slowing of chronic kidney disease progression than with a maximal clinical dose of either drug alone, even without an additional reduction in blood pressure.39 However, the findings are limited to patients with IgA nephropathy and as yet have not been confirmed in patients with chronic kidney disease due to hypertension and diabetes. Studies are underway to test effects of a combined ACE Inhibitor/ARB strategy on cardiovascular outcomes.40
RENIN-ANGIOTENSIN SYSTEM INHIBITION FOR DIABETES PREVENTION?
Inappropriate renin-angiotensin system activation has been implicated in glucose intolerance by a variety of angiotensin II type 1 receptor mechanisms including impaired adipocyte differentiation, pancreatic β-cell function, and insulin-mediated glucose disposal.14, 15 A retrospective meta-analysis of mainly hypertension trials suggested that ACE Inhibitors and ARBs consistently reduce new cases of type 2 diabetes.10 However, the only prospective study on this topic, the recent Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial, failed to show that ramipril prevented or delayed the onset of type 2 diabetes or death in patients with impaired fasting glucose or glucose intolerance.41 Ramipril’s disappointing performance may be related to a low incidence of baseline cardiovascular disease in the study population and thus presumably a low baseline level of renin-angiotensin system activation, the relatively short 3-year duration of the trial (compared to 5-year duration of most hypertension trials), and incomplete renin-angiotensin system blockade.
By providing more complete renin-angiotensin system blockade than with ACE Inhibitors and ARBs, the hope is that direct renin inhibitors will afford greater protection against the cardiovascular, renal, and metabolic complications associated with hypertension.
DIRECT RENIN INHIBITION: A NOVEL MECHANISM OF ACTION
A fundamental reason why ACE Inhibitors and ARBs produce incomplete renin-angiotensin system blockade is reduced feedback inhibition of renin release, triggering a reactive rise in plasma renin activity.42, 43 With an ACE Inhibitor, the reactive rise in plasma renin activity causes a compensatory increase in angiotensin I which partially restores angiotensin II production by ACE-dependent and ACE-independent pathways (Figure 3). With an ARB, the reactive rise in plasma renin activity causes compensatory increases in both angiotensin I and angiotensin II. The latter may partially restore angiotensin II type 1 receptor stimulation and stimulate possibly profibrotic angiotensin type 2 receptors and prothrombotic angiotensin type 4 receptors (Figure 3).25
Figure 3.
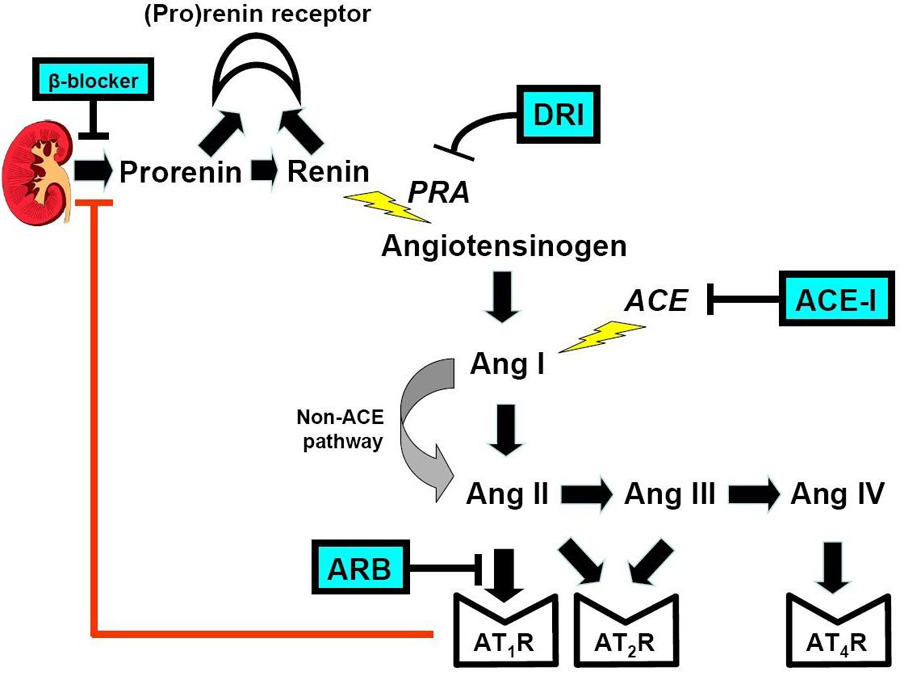
Site of action of four classes of antihypertensive drugs that block the renin-angiotensin system: beta-blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and the direct renin inhibitor.
DRI, direct renin inhibitor; PRA, plasma renin activity; ACEI, angiotensin converting enzyme inhibitor; Ang III, angiotensin III; Ang IV, angiotensin IV; ARB, angiotensin receptor blocker; AT2R, angiotensin type 2 receptor; AT4R, angiotensin type 4 receptor.
In contrast, the direct renin inhibitor binds directly to the catalytic site of renin, thereby inhibiting its ability to convert angiotensinogen to angiotensin I, the rate limiting step in the formation of angiotensin II (Figure 3).44, 45 Because of this proximal renin-angiotensin system blockade, direct renin inhibitor therapy is accompanied by decreased angiotensin I and angiotensin II levels.42 The resultant loss of feedback inhibition elicits a large reactive rise in renin secretion but plasma renin activity—the enzymatic activity of renin—is markedly reduced by the direct renin inhibitor.42 Thus, while plasma renin activity, angiotensin I and angiotensin II all increase reflexively with ARBs, they decrease markedly with a direct renin inhibitor (Figure 4).42
Figure 4.
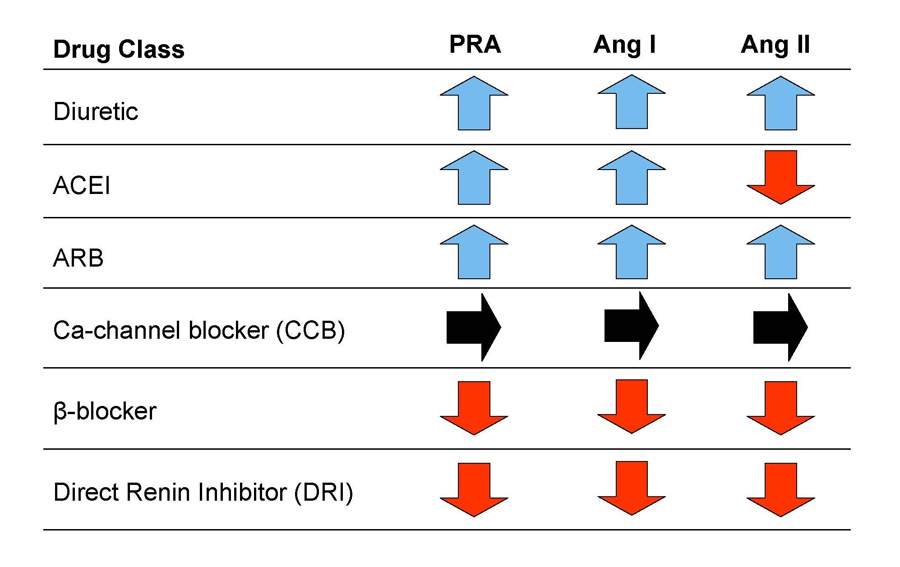
Comparative effects of different drug classes on plasma renin activity, serum angiotensin I, and serum angiotensin II levels.
Ca, calcium
POTENTIAL ADVANTAGES OF ALISKIREN
Aliskiren provides a critically important new opportunity to test whether greater renin-angiotensin system inhibition will translate into greater blood pressure-independent end-organ protection than that provided by ACE Inhibitors and ARBs. A key question will be whether for any given blood pressure reduction, will target organ protection be greater with the direct renin inhibitor than with an ACE Inhibitor or ARB, or when the direct renin inhibitor is added to pre-existing ACE Inhibitor/ARB therapy.
Indeed, aliskiren’s novel mechanism of action makes it particularly well suited to be used in combination with antihypertensives that cause a reactive rise in plasma renin activity—diuretics, ACE Inhibitors, and ARBs.7 As would be expected, addition of aliskiren to a thiazide diuretic causes an additional fall in blood pressure.46 A much more interesting and potentially important finding is that addition of aliskiren to an ARB has recently been shown to cause an additional fall in blood pressure.47 In a double-blind study, 1797 patients with fairly severe hypertension were randomized to 8-weeks of treatment with placebo, aliskiren alone, valsartan alone, or the combination. The combination of aliskiren and valsartan at maximal recommended doses (300 and 320 mg, respectively) yielded a greater fall in both clinic and ambulatory blood pressure than either drug alone (Figure 5). This finding differentiates aliskiren from ACE Inhibitors as combined ACE Inhibitor/ARB therapy does not yield a greater fall in blood pressure than a maximal dose of either drug alone.39
Figure 5.
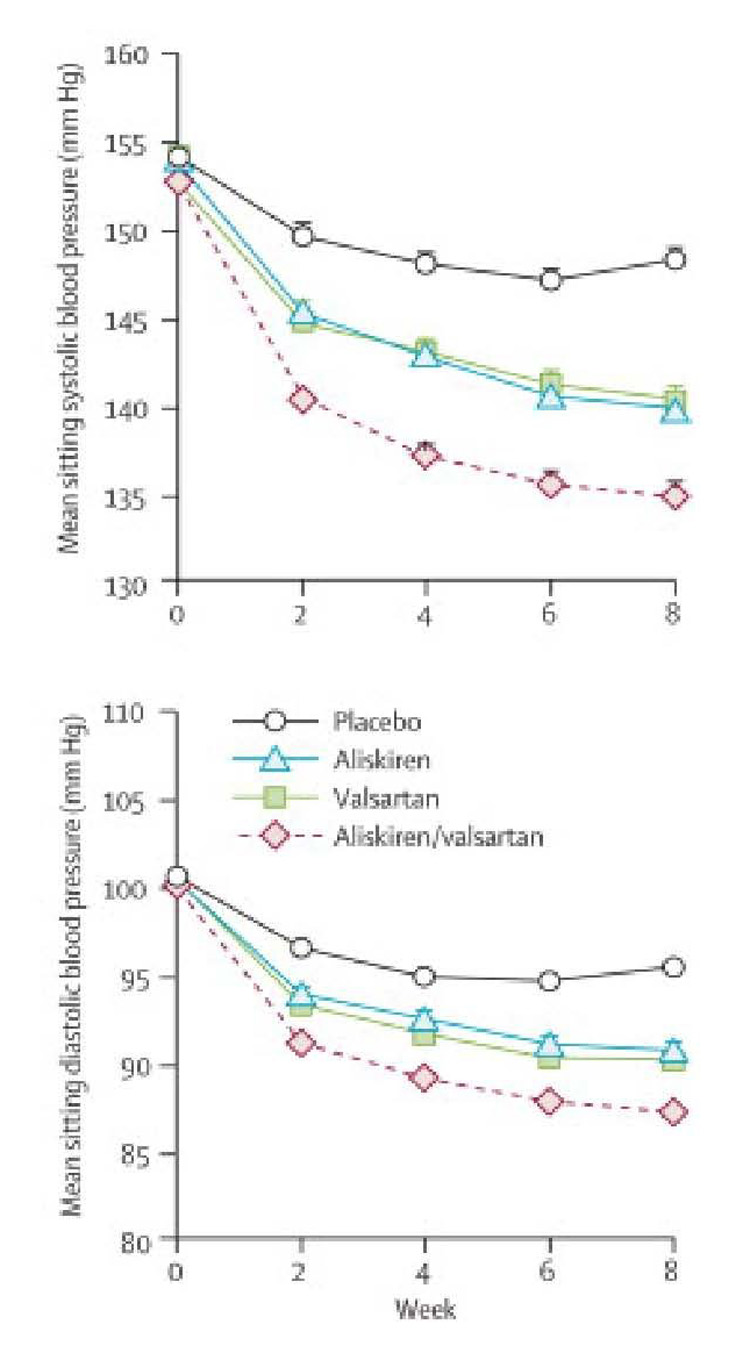
Effect of placebo, aliskiren, valsartan, and aliskiren/valsartan combination therapy on mean sitting systolic and diastolic blood pressure.
mm Hg, millimeters mercury
Modified with permission from: Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007 July 21;370(9583):221–9.
In the doses marketed (150 and 300 mg per day), aliskiren has thus far been very well tolerated with the same placebo-like side-effect profile as the ARBs. It also does not inhibit bradykinin metabolism and does not cause a cough.
The high affinity binding to renin concentrates aliskiren in the kidney, a unique property that holds particular promise for renal protection. The avid renal concentration may well explain the long half-life of > 24 hours and the slow recovery of blood pressure after the drug has been discontinued.48 These properties should promote continuous 24h blood pressure control.
POTENTIAL LIMITATIONS OF ALISKIREN
As monotherapy aliskiren appears to be equally effective as an ACE Inhibitor or ARB in lowering blood pressure, but not more effective.43, 49–51 On average, ambulatory blood pressure falls by ~10/7 mmHg.47, 51 Why is it that blood pressure does not fall more with the more complete renin-angiotensin system inhibition provided by the direct renin inhibitor? Several explanations can be proposed,4 but the simplest is that the renin-angiotensin system is only one of many regulatory pathways contributing to hypertension. The modest blood pressure reduction with aliskiren monotherapy most likely reflects compensation. Regardless of the precise explanation, aliskiren is not going to eliminate the need for multi-drug regimens to control most cases of hypertension, nor is it expected to be a magic bullet for resistant hypertension.
There are other limitations that should be noted. The maximal daily dose is capped at 300 mg because higher doses can produce diarrhea, which is presumably due to 98% of the drug not being absorbed from the gut.2 Absorption is further reduced if aliskiren is taken with a fatty meal, thereby compromising bioavailability.52 When aliskiren is co-administered with furosemide, the peak furosemide blood concentration is reduced by as much as 50%.52 As with all renin-angiotensin system inhibitors, serum potassium needs to be monitored regularly and pregnancy is an absolute contraindication.
From a theoretical standpoint, two potential limitations could arise from aliskiren’s novel mechanism of action. First, the proximal renin-angiotensin system blockade reduces the concentration of multiple downstream intermediates that in rodent models have both accelerated and retarded hypertensive target organ disease.9, 16, 25, 53, 54 The net effect in patients is unknown.
The second and more intriguing mechanistic consideration relates to renin secretion. With aliskiren, the reactive rise in renin secretion is greater than with an ACE Inhibitor or ARB, reflecting more complete renin-angiotensin system inhibition.4, 47 When aliskiren is combined with HCTZ or an ARB, the reactive rise in renin secretion is dramatic.4, 47 This compensation could limit the absolute decrease in plasma renin activity and attendant fall in blood pressure, because the enzymatic block by the direct renin inhibitor is not 100% but closer to 75%.4 Furthermore, high circulating levels of renin—and its precursor (prorenin)—may activate fibrotic signaling pathways by novel receptor-mediated mechanisms that are completely independent of angiotensin II production and angiotensin II type 1 receptor stimulation.55–57
POTENTIAL EFFECTS OF ALISKIREN ON (PRO)RENIN RECEPTOR ACTIVATION
An emerging body of basic research indicates that prorenin can no longer be considered merely the inactive precursor of renin. Secreted by the juxtaglomerular cells, prorenin initially is inactive because a hinge covers the angiotensinogen binding site (Figure 6).44 Classically, prorenin is converted to renin by enzymatic cleavage of the hinge, thereby exposing the angiotensinogen catalytic site. The catalytic site can be exposed and prorenin fully activated by a non-enzymatic process in which the hinge swings open but is not cleaved.44 This process occurs when circulating prorenin binds to a recently discovered class of (pro)renin receptors in the heart, kidneys, and presumably other target organs.44, 58 By blocking the catalytic site of both renin and activated prorenin, the direct renin inhibitor should eliminate the generation of angiotensin I both in the systemic circulation and at the tissue level (Figure 6). Because prorenin circulates at levels that are 10–100 times higher than renin,44 prorenin inhibition holds promise for enhanced end-organ protection.
Figure 6.
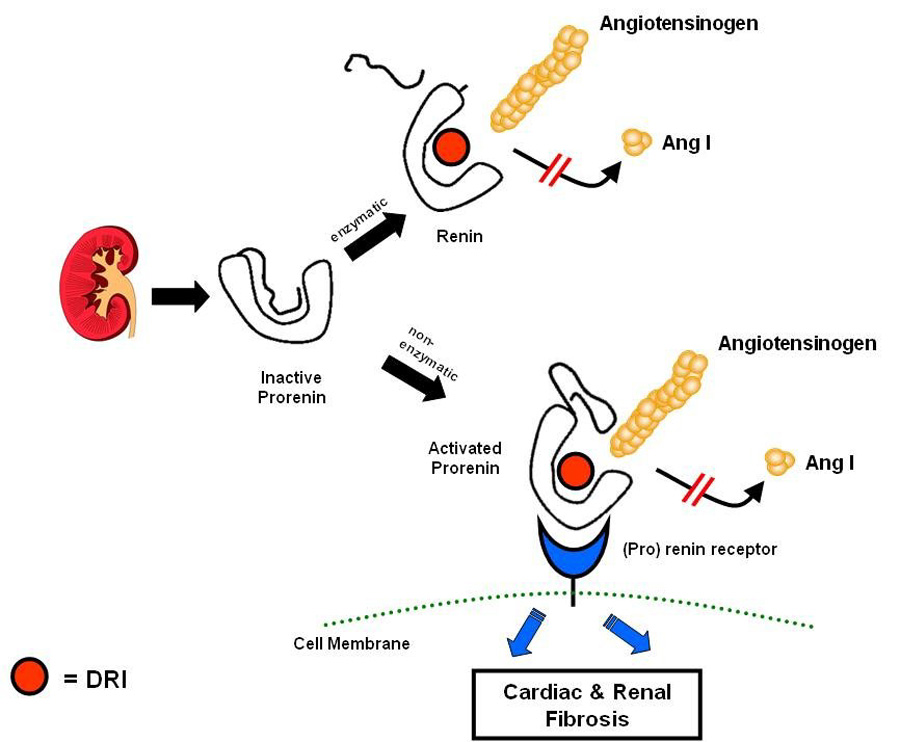
Inhibition of both enzymatic and nonenzymatic activation of prorenin.
Modified with permission from: Danser AH, Deinum J. Renin, prorenin and the putative (pro)renin receptor. Hypertension 2005 November;46(5):1069–76.
However, (pro)renin receptor activation also has been shown in rodents to stimulate profibrotic pathways in the heart and kidney that are completely independent of angiotensin II and therefore unaffected by ACE Inhibitors and ARBs—and resumably unaffected by a direct renin inhibitor.55–57 Because the reactive rise in pro(renin) secretion is greater with the direct renin inhibitor than with an ACE Inhibitor or ARB—and extremely high when the direct renin inhibitor is combined with a thiazide or ARB—activation of fibrotic pathways is a theoretical concern. This concern is potentially mitigated by a recent study suggesting that high concentrations of renin and prorenin evoke feedback suppression of receptor expression.59 Clearly, this is a rapidly evolving field of intense research with many unanswered questions, the most important relating to clinical translation. If, for example, the reactive rise in renin secretion turns out to limit aliskiren’s clinical benefits, a logical next step would be to examine combination therapy with beta-blockers, the only drugs known to block renin secretion.
CONCLUSIONS
As with any new drug class, the true contribution of direct renin inhibitors to medical therapeutics awaits outcomes of large randomized trials and years of clinical experience. While aliskiren adds to a list of > 100 drugs approved for the treatment of hypertension, its introduction into clinical medicine is of particular interest because of the novel mechanism of action. Until outcomes data become available, there is as yet no evidence base to justify replacing an ACE Inhibitor or ARB with aliskiren for end-organ protection in patients with heart failure, chronic kidney disease, or diabetes. Aliskiren already represents an attractive new option as add-on therapy for achieving blood pressure goals in a higher percentage of our patients.
Acknowledgments
Financial Support Statement: We wish to acknowledge the following sources of funding: National Institutes of Health (NIH) training grant in cardiovascular disease, T32-HL07360-29 (Dr. Shafiq), American Society of Hypertension Fellowship grant (Dr. Menon); and NIH RO-1 HL044010 and the Donald W. Reynolds Foundation (Dr. Victor).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement (from all authors): Moiz Shafiq, MD – none
Dileep Menon, MD – none
Ronald Victor, MD – Dr. Victor is on the Scientific Advisory Board of Novartis and cardiovascularRx, and is the recipient of research grants from Pfizer and Aetna Foundation.
Reference List
- 1.Azizi M, Webb R, Nussberger J, Hollenberg NK. Renin inhibition with aliskiren: where are we now, and where are we going. J Hypertens. 2006 February;24(2):243–256. doi: 10.1097/01.hjh.0000202812.72341.99. [DOI] [PubMed] [Google Scholar]
- 2.Staessen JA, Li Y, Richart T. Oral renin inhibitors. Lancet. 2006 October 21;368(9545):1449–1456. doi: 10.1016/S0140-6736(06)69442-7. [DOI] [PubMed] [Google Scholar]
- 3.Stanton A. Therapeutic potential of renin inhibitors in the management of cardiovascular disorders. Am J Cardiovasc Drugs. 2003;3(6):389–394. doi: 10.2165/00129784-200303060-00002. [DOI] [PubMed] [Google Scholar]
- 4.Sealey JE, Laragh JH. Aliskiren, the first renin inhibitor for treating hypertension: reactive renin secretion may limit its effectiveness. Am J Hypertens. 2007 May;20(5):587–597. doi: 10.1016/j.amjhyper.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun DA. Use of aldosterone antagonists in resistant hypertension. Prog Cardiovasc Dis. 2006 May;48(6):387–396. doi: 10.1016/j.pcad.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien E, Barton J, Nussberger J, et al. Aliskiren Reduces Blood Pressure and Suppresses Plasma Renin Activity in Combination With a Thiazide Diuretic, an Angiotensin-Converting Enzyme Inhibitor, or an Angiotensin Receptor Blocker. Hypertension. 2006 December 11; doi: 10.1161/01.HYP.0000253780.36691.4f. [DOI] [PubMed] [Google Scholar]
- 8.Siragy HM, Xue C, Abadir P, Carey RM. Angiotensin subtype-2 receptors inhibit renin biosynthesis and angiotensin II formation. Hypertension. 2005 January;45(1):133–137. doi: 10.1161/01.HYP.0000149105.75125.2a. [DOI] [PubMed] [Google Scholar]
- 9.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006 July 15;71(2):247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Abuissa H, Jones PG, Marso SP, O'Keefe JH., Jr Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol. 2005 September 6;46(5):821–826. doi: 10.1016/j.jacc.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 11.Candido R, Allen TJ, Lassila M, et al. Irbesartan but not amlodipine suppresses diabetes-associated atherosclerosis. Circulation. 2004 March 30;109(12):1536–1542. doi: 10.1161/01.CIR.0000124061.78478.94. [DOI] [PubMed] [Google Scholar]
- 12.Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006 April;259(4):351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 13.Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003 July;115(1):41–46. doi: 10.1016/s0002-9343(03)00158-x. [DOI] [PubMed] [Google Scholar]
- 14.Lau T, Carlsson PO, Leung PS. Evidence for a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia. 2004 February;47(2):240–248. doi: 10.1007/s00125-003-1295-1. [DOI] [PubMed] [Google Scholar]
- 15.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006 April 4;144(7):517–524. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- 16.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001 January 23;103(3):448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 17.Zhou MS, Jaimes EA, Raij L. Inhibition of oxidative stress and improvement of endothelial function by amlodipine in angiotensin II-infused rats. Am J Hypertens. 2004 February;17(2):167–171. doi: 10.1016/j.amjhyper.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 18.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987 June 4;316(23):1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 19.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995 May 10;273(18):1450–1456. [PubMed] [Google Scholar]
- 20.Investigators TS. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 21.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003 September 6;362(9386):759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 22.Pitt B, Segal R, Martinez FA, et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE) Lancet. 1997 March 15;349(9054):747–752. doi: 10.1016/s0140-6736(97)01187-2. [DOI] [PubMed] [Google Scholar]
- 23.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001 December 6;345(23):1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 24.Sever PS, Poulter NR, Elliott WJ, et al. Blood pressure reduction is not the only determinant of outcome. Circulation. 2006 June 13;113(23):2754–2772. doi: 10.1161/CIRCULATIONAHA.105.588020. [DOI] [PubMed] [Google Scholar]
- 25.Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006 August 22;114(8):838–854. doi: 10.1161/CIRCULATIONAHA.105.594986. [DOI] [PubMed] [Google Scholar]
- 26.Verdecchia P, Reboldi G, Angeli F, et al. Angiotensin-converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention. Hypertension. 2005 August;46(2):386–392. doi: 10.1161/01.HYP.0000174591.42889.a2. [DOI] [PubMed] [Google Scholar]
- 27.Staessen JA, Li Y, Thijs L, Wang JG. Blood pressure reduction and cardiovascular prevention: an update including the 2003–2004 secondary prevention trials. Hypertens Res. 2005 May;28(5):385–407. doi: 10.1291/hypres.28.385. [DOI] [PubMed] [Google Scholar]
- 28.Wang JG, Li Y, Franklin SS, Safar M. Prevention of stroke and myocardial infarction by amlodipine and Angiotensin receptor blockers: a quantitative overview. Hypertension. 2007 July;50(1):181–188. doi: 10.1161/HYPERTENSIONAHA.107.089763. [DOI] [PubMed] [Google Scholar]
- 29.Tsuyuki RT, McDonald MA. Angiotensin receptor blockers do not increase risk of myocardial infarction. Circulation. 2006 August 22;114(8):855–860. doi: 10.1161/CIRCULATIONAHA.105.594978. [DOI] [PubMed] [Google Scholar]
- 30.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004 June 19;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 31.Julius S, Weber MA, Kjeldsen SE, et al. The Valsartan Antihypertensive Long-Term Use Evaluation (VALUE) trial: outcomes in patients receiving monotherapy. Hypertension. 2006 September;48(3):385–391. doi: 10.1161/01.HYP.0000236119.96301.f2. [DOI] [PubMed] [Google Scholar]
- 32.Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004 June 19;363(9426):2049–2051. doi: 10.1016/S0140-6736(04)16456-8. [DOI] [PubMed] [Google Scholar]
- 33.Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285(21):2719–2728. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]
- 34.Chiurchiu C, Remuzzi G, Ruggenenti P. Angiotensin-converting enzyme inhibition and renal protection in nondiabetic patients: the data of the meta-analyses. J Am Soc Nephrol. 2005 March;16 Suppl 1:S58–S63. doi: 10.1681/asn.2004110968. [DOI] [PubMed] [Google Scholar]
- 35.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 36.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 37.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 38.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 39.Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003 January 11;361(9352):117–124. doi: 10.1016/S0140-6736(03)12229-5. [DOI] [PubMed] [Google Scholar]
- 40.Teo K, Yusuf S, Sleight P, et al. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. Am Heart J. 2004 July;148(1):52–61. doi: 10.1016/j.ahj.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006 October 12;355(15):1551–1562. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- 42.Nussberger J, Wuerzner G, Jensen C, Brunner HR. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension. 2002 January;39(1):E1–E8. doi: 10.1161/hy0102.102293. [DOI] [PubMed] [Google Scholar]
- 43.Stanton A, Jensen C, Nussberger J, O'Brien E. Blood pressure lowering in essential hypertension with an oral renin inhibitor, aliskiren. Hypertension. 2003 December;42(6):1137–1143. doi: 10.1161/01.HYP.0000101688.17370.87. [DOI] [PubMed] [Google Scholar]
- 44.Danser AH, Deinum J. Renin, prorenin and the putative (pro)renin receptor. Hypertension. 2005 November;46(5):1069–1076. doi: 10.1161/01.HYP.0000186329.92187.2e. [DOI] [PubMed] [Google Scholar]
- 45.Wood JM, Maibaum J, Rahuel J, et al. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun. 2003 September 5;308(4):698–705. doi: 10.1016/s0006-291x(03)01451-7. [DOI] [PubMed] [Google Scholar]
- 46.Villamil A, Chrysant SG, Calhoun D, et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens. 2007 January;25(1):217–226. doi: 10.1097/HJH.0b013e3280103a6b. [DOI] [PubMed] [Google Scholar]
- 47.Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet. 2007 July 21;370(9583):221–229. doi: 10.1016/S0140-6736(07)61124-6. [DOI] [PubMed] [Google Scholar]
- 48.Zhao C, Vaidyanathan S, Yeh CM, Maboudian M, Armin DH. Aliskiren exhibits similar pharmacokinetics in healthy volunteers and patients with type 2 diabetes mellitus. Clin Pharmacokinet. 2006;45(11):1125–1134. doi: 10.2165/00003088-200645110-00006. [DOI] [PubMed] [Google Scholar]
- 49.Azizi M, Menard J, Bissery A, et al. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol. 2004 December;15(12):3126–3133. doi: 10.1097/01.ASN.0000146686.35541.29. [DOI] [PubMed] [Google Scholar]
- 50.Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation. 2005 March 1;111(8):1012–1018. doi: 10.1161/01.CIR.0000156466.02908.ED. [DOI] [PubMed] [Google Scholar]
- 51.Oh BH, Mitchell J, Herron JR, Chung J, Khan M, Keefe DL. Aliskiren, an oral renin inhibitor, provides dose-dependent efficacy and sustained 24-hour blood pressure control in patients with hypertension. J Am Coll Cardiol. 2007 March 20;49(11):1157–1163. doi: 10.1016/j.jacc.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 52.Novartis Pharmaceuticals Corporation. Tekturna (aliskiren) Prescribing Information. 2007
- 53.Carey RM. Cardiovascular and renal regulation by the angiotensin type 2 receptor: the AT2 receptor comes of age. Hypertension. 2005 May;45(5):840–844. doi: 10.1161/01.HYP.0000159192.93968.8f. [DOI] [PubMed] [Google Scholar]
- 54.Ferrario CM, Jessup J, Gallagher PE, et al. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int. 2005 November;68(5):2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 55.Ichihara A, Kaneshiro Y, Takemitsu T, et al. Contribution of nonproteolytically activated prorenin in glomeruli to hypertensive renal damage. J Am Soc Nephrol. 2006 September;17(9):2495–2503. doi: 10.1681/ASN.2005121278. [DOI] [PubMed] [Google Scholar]
- 56.Ichihara A, Suzuki F, Nakagawa T, et al. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2006 July;17(7):1950–1961. doi: 10.1681/ASN.2006010029. [DOI] [PubMed] [Google Scholar]
- 57.Ichihara A, Kaneshiro Y, Takemitsu T, et al. Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension. 2006 May;47(5):894–900. doi: 10.1161/01.HYP.0000215838.48170.0b. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen G. Renin/prorenin receptors. Kidney Int. 2006 May;69(9):1503–1506. doi: 10.1038/sj.ki.5000265. [DOI] [PubMed] [Google Scholar]
- 59.Schefe JH, Menk M, Reinemund J, et al. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006 December 8;99(12):1355–1366. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]


