Abstract
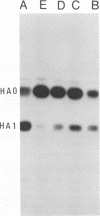
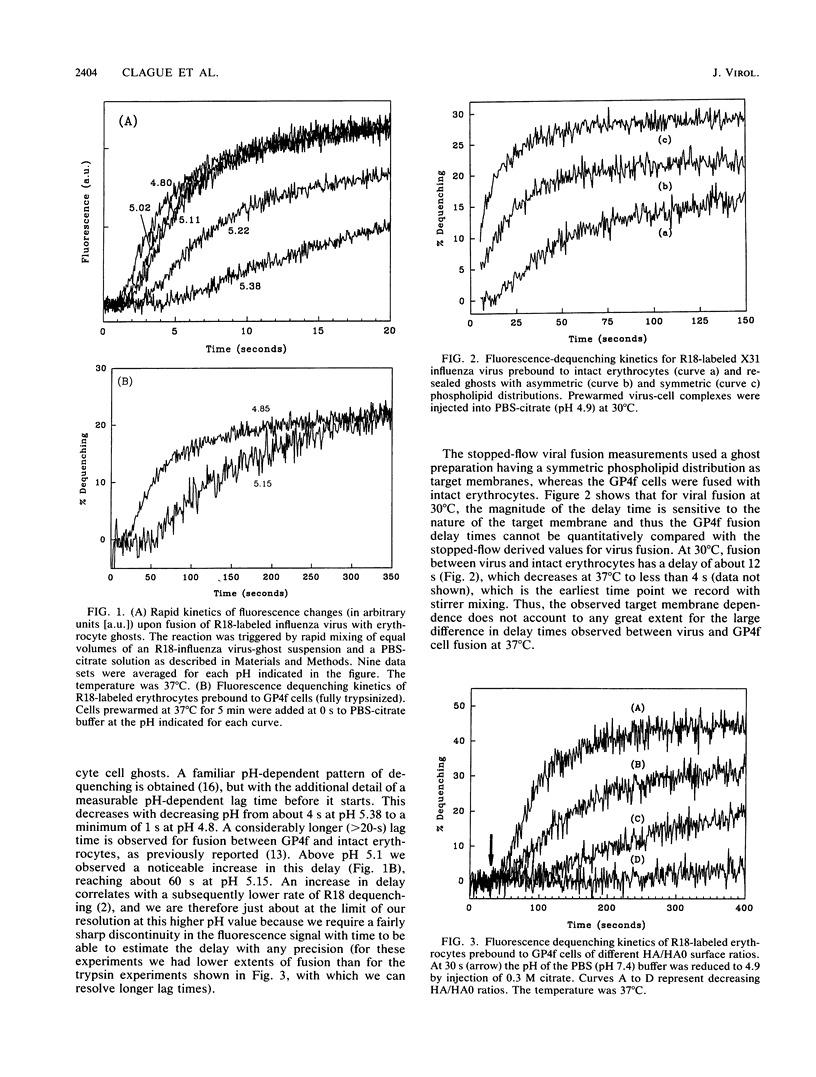
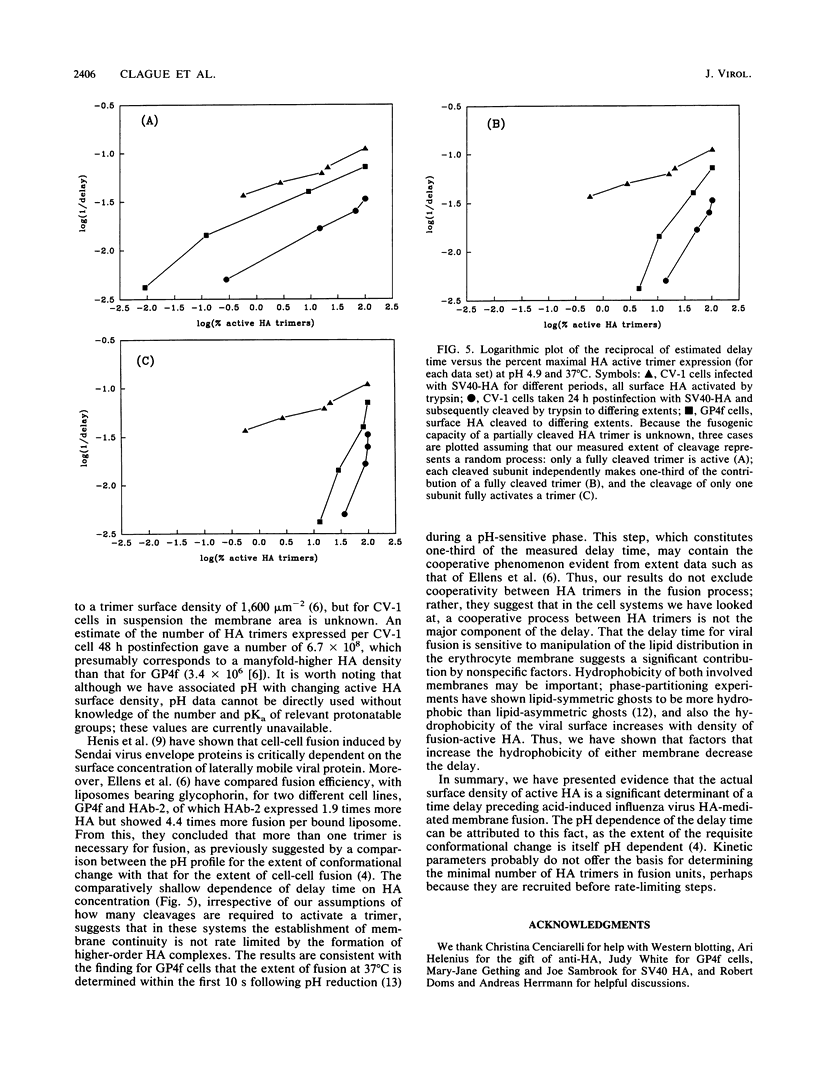
We have studied the kinetics of low-pH-induced fusion between erythrocyte membranes and membranes containing influenza virus hemagglutinin by using assays based on the fluorescence dequenching of the lipophilic dye octadecylrhodamine. Stopped-flow mixing and fast data acquisition have been used to monitor the early stages of influenza virus fusion. We have compared this with the kinetics observed for fusion of an NIH 3T3 cell line, transformed with bovine papillomavirus, which constitutively expresses influenza virus hemagglutinin (GP4f cells). Virus and GP4f cells both display a pH-dependent time lag before the onset of fluorescence dequenching, but of an order of magnitude difference, ca. 2 s versus ca. 20 s. We have adopted two strategies to investigate whether the difference in lag time reflects the surface density of acid-activated hemagglutinin, able to undergo productive conformational change. (i) Hemagglutinin expressed on the cell surface requires proteolytic cleavage with trypsin from an inactive HAO form; we have limited the extent of proteolysis. (ii) We have used infection of CV-1 cells with a recombinant simian virus 40 bearing the influenza virus hemagglutinin gene. The surface expression of hemagglutinin is a function of time postinfection. For low-pH-induced fusion of both types of cell with erythrocytes, the lag time decreases with increasing hemagglutinin densities. Our results do not indicate a cooperative phenomenon at the level of the principal rate-determining step. We also show in the instance of virus fusion, that the magnitude of the delay time is a function of the target membrane transbilayer lipid distribution. We conclude that for a given amount of pH-activated hemagglutinin per unit area of membrane, the kinetics of fusion is determined by nonspecific physical properties of the membranes involved.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulay F., Doms R. W., Webster R. G., Helenius A. Posttranslational oligomerization and cooperative acid activation of mixed influenza hemagglutinin trimers. J Cell Biol. 1988 Mar;106(3):629–639. doi: 10.1083/jcb.106.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague M. J., Schoch C., Zech L., Blumenthal R. Gating kinetics of pH-activated membrane fusion of vesicular stomatitis virus with cells: stopped-flow measurements by dequenching of octadecylrhodamine fluorescence. Biochemistry. 1990 Feb 6;29(5):1303–1308. doi: 10.1021/bi00457a028. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Helenius A., White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem. 1985 Mar 10;260(5):2973–2981. [PubMed] [Google Scholar]
- Doxsey S. J., Sambrook J., Helenius A., White J. An efficient method for introducing macromolecules into living cells. J Cell Biol. 1985 Jul;101(1):19–27. doi: 10.1083/jcb.101.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Mason D., Zhang F., White J. M. Fusion of influenza hemagglutinin-expressing fibroblasts with glycophorin-bearing liposomes: role of hemagglutinin surface density. Biochemistry. 1990 Oct 16;29(41):9697–9707. doi: 10.1021/bi00493a027. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Doms R. W., York D., White J. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J Cell Biol. 1986 Jan;102(1):11–23. doi: 10.1083/jcb.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Cell-surface expression of influenza haemagglutinin from a cloned DNA copy of the RNA gene. Nature. 1981 Oct 22;293(5834):620–625. doi: 10.1038/293620a0. [DOI] [PubMed] [Google Scholar]
- Henis Y. I., Herman-Barhom Y., Aroeti B., Gutman O. Lateral mobility of both envelope proteins (F and HN) of Sendai virus in the cell membrane is essential for cell-cell fusion. J Biol Chem. 1989 Oct 15;264(29):17119–17125. [PubMed] [Google Scholar]
- Hoekstra D., de Boer T., Klappe K., Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984 Nov 20;23(24):5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983 Aug;34(1):233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- McEvoy L., Williamson P., Schlegel R. A. Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc Natl Acad Sci U S A. 1986 May;83(10):3311–3315. doi: 10.1073/pnas.83.10.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. J., Sarkar D. P., White J. M., Blumenthal R. Kinetics of pH-dependent fusion between 3T3 fibroblasts expressing influenza hemagglutinin and red blood cells. Measurement by dequenching of fluorescence. J Biol Chem. 1989 Mar 5;264(7):3972–3978. [PubMed] [Google Scholar]
- Sarkar D. P., Morris S. J., Eidelman O., Zimmerberg J., Blumenthal R. Initial stages of influenza hemagglutinin-induced cell fusion monitored simultaneously by two fluorescent events: cytoplasmic continuity and lipid mixing. J Cell Biol. 1989 Jul;109(1):113–122. doi: 10.1083/jcb.109.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Bayley P. M., Brown E. B., Martin S. R., Waterfield M. D., White J. M., Wilson I. A., Wiley D. C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982 Feb;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T., Hoekstra D., Scherphof G., Wilschut J. Fusion activity of influenza virus. A comparison between biological and artificial target membrane vesicles. J Biol Chem. 1986 Aug 25;261(24):10966–10969. [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Williamson P., Algarin L., Bateman J., Choe H. R., Schlegel R. A. Phospholipid asymmetry in human erythrocyte ghosts. J Cell Physiol. 1985 May;123(2):209–214. doi: 10.1002/jcp.1041230209. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Kuroda K., Kawasaki K., Yamashina S., Maeda T., Ohnishi S. Infectious cell entry mechanism of influenza virus. J Virol. 1982 Jul;43(1):284–293. doi: 10.1128/jvi.43.1.284-293.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]