Abstract
Alpha-catenin is a structural molecule and essential to the function of epithelial adherens junctions. Its role in the morphogenesis of mammary epithelium was explored using experimental mouse genetics. Since loss of α-catenin in mice leads to embryonic lethality, the α-catenin gene was flanked by loxP sites and inactivated in mammary epithelium using the WAP-Cre and MMTV-Cre transgenes. Loss of α-catenin arrested alveolar epithelial expansion. These cells lacked proper polarity and markers of functional differentiation, which resulted in impaired milk protein gene expression. Without α-catenin, increased epithelial cell death was observed at parturition and the tissue resembled an involuted gland that is normally observed after weaning. Lastly, no tumors were detected in mammary tissue lacking α-catenin.
Keywords: Mammary development, Alpha-catenin, WAP-Cre, MMTV-Cre, Conditional deletion
1. Introduction
The lactating mammary gland consists of two distinct epithelial compartments, a branching system of ducts embedded in connective tissue and a lobuloalveolar structure containing differentiated secretory epithelial cells. The epithelial cells are surrounded by a network of myoepithelial cells. Mammary gland morphogenesis proceeds in distinct phases. A mammary anlage and rudimentary ducts form in the fetus and grow isometrically until puberty. After the onset of puberty, the majority of ductal elongation and branching occurs. Pregnancy induces alveolar proliferation in which functional differentiation and milk secretion are attained in response to the synergistic action of lactogenic hormones and local growth factors (Hennighausen and Robinson, 1998, 2001; Neville, 1999). At the end of the lactation period, involution ensues and the mammary alveolar compartment is remodeled to resemble that of a mature virgin so that during the following pregnancy a new cycle of development may be initiated. Throughout this dynamic cycle of alveolar proliferation, functional differentiation, milk secretion and cell death, it is crucial for the epithelial cells of the mammary gland to maintain their integrity.
In part, epithelial integrity is maintained by adherens junctions, which are intercellular structures necessary for cell-to-cell contact. The epithelial type of adherens junctions is composed of the E-cadherin/catenin complex. This complex consists of transmembrane E-cadherin and its associated intracellular catenins (α, β, γ, and p120) (Beavon, 2000). The extracellular region of E-cadherin is responsible for homotypic interactions facilitating cell–cell connections, while the cytoplasmic domain of E-cadherin binds to β-catenin. Beta-catenin binds to α-catenin, which is required to anchor the cadherin complex to the actin cytoskeleton (Beavon, 2000). The importance of α-catenin in cell–cell adhesion, polarization, cytoskeletal, stabilization and function of epithelial cells has been established, to some extent, in normal development (Nanba et al., 2001) and tumor tissues (Van Aken et al., 2001). Inactivation of α-catenin in mice causes embryonic lethality due to disruption of the trophoblast epithelium (Torres et al. 1997). Cell-specific ablation of α-catenin in the skin of mice results in hyperproliferative and invasive characteristics in the epidermis of embryos (Vasioukhin et al., 2001). In patient tumor tissues, a reduction or loss of α-catenin expression has been reported widely, thus implicating this molecule in tumor progression and metastasis (Van Aken et al., 2001). In breast cancer tissue samples, it has been reported that primary tumors lose E-cadherin and catenin complexes only to re-establish themselves in metastases (Bukholm et al., 1998, 2000). These data have led to the hypothesis that α-catenin may have a role in both cell adhesion and suppression of metastatic progression in mammary epithelium.
This study addresses the contributions of α-catenin in the developing mammary epithelium and as a tumor suppressor as suggested in skin. To avoid the embryonic lethality associated with α-catenin deficient mice, the gene encoding α-catenin was inactivated using Cre-loxP mediated recombination. For this purpose, Cre transgenes were bred into mice that carried the α-catenin gene targeted with loxP sites (Vasioukhin et al., 2001). The Cre expression under control of the whey acidic protein (WAP) gene promoter is active in mammary epithelium during the second half of pregnancy upon the initiation of differentiation (Wagner et al., 1997). In contrast, Cre expression under control of the mouse mammary tumor virus long terminal repeat (MMTV-LTR) (Wagner et al., 1997, 2001) is present in mammary epithelium during embryogenesis. In these mice, Cre is expressed in mammary ductal and alveolar epithelium in addition to several other tissues throughout development.
2. Results
2.1. Normal mammary epithelium and stroma express α-catenin
Mammary tissue from C57BL/6 mice was analyzed at various stages of development for the presence of α-catenin and E-cadherin (Fig. 1). Alpha-catenin was expressed throughout all stages of mammary development, i.e. in the mature virgin, during pregnancy, lactation, and involution. The cleared mammary fat pad, which is devoid of epithelium, also expressed α-catenin indicating its presence in the mammary stroma. E-cadherin was found only in mammary tissue containing epithelium and not in the stroma.
Fig. 1.

Expression of α-catenin and E-cadherin in mouse mammary tissue. Western blot analysis of α-catenin and E-cadherin in wild type mammary tissues at various developmental stages: lane 1, epithelium cleared mammary fat pad; lane 2, 10-week virgin; lanes 3–5, pregnancy days 11,14 and 18; lanes 6-7, lactation days 1 and 10; lanes 8-9, involution days 2 and 10.
2.2. Alpha-catenin is required for alveolar expansion
Ductal elongation and branching were unaffected upon inactivation of the α-catenin gene using the MMTV-Cre transgenic mice (data not shown). Since the MMTV-Cre transgene is expressed in several distinct cell types, phenotypic consequences due to the loss of α-catenin had been expected. However, these mice appeared normal with the exception of an occasional lack of scheduled hair development (data not shown).
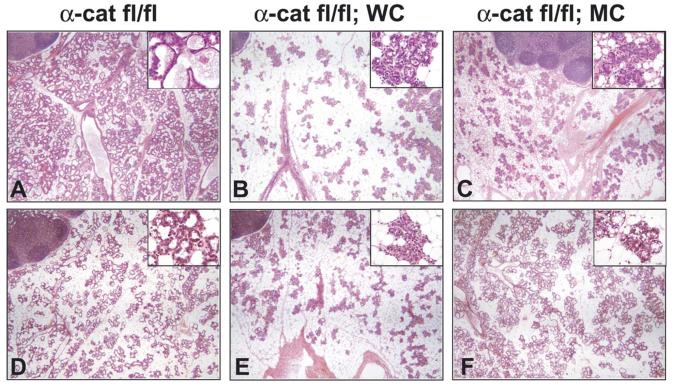
In mammary glands from α-catenin fl/fl (α-cat fl/fl) controls at parturition, alveoli formed normally with polarized epithelium and expanded lumina, which displayed active milk and lipid secretion (Fig. 2A). In contrast, a paucity of epithelium was observed throughout the gland in α-cat fl/fl;WC (WC; WAP-Cre) and α-cat fl/fl;MC (MC; MMTV-Cre) mice at parturition (Fig. 2B and C, respectively). The epithelial cells in these glands were grouped within two distinct populations. One population of epithelial cells had retained the ability to form alveoli of normal appearance that produced lipids, while the second population of cells failed to form alveolar structures and existed as disorganized, condensed islands of epithelial cell clusters throughout the stroma (Fig. 2B and C insets). The presence of the second population of epithelial clusters, which had no identifiable central lumina and no evidence of lipid droplets, suggested an impaired differentiation program. The heterogeneity of this tissue can be attributed to the mosaic expression of WAP-Cre and MMTV-Cre transgene expression (Wagner et al., 2001). Accordingly, some dams were able to support their litters while others were not able to lactate at all.
Fig. 2.
Hematoxylin and eosin staining of mammary biopsies at parturition. (A) Section from α-catenin fl/fl control tissue in which alveoli were expanded and milk and milk fat secretion were evident. (B, C) Sections from α-cat fl/fl;WC and α-cat fl/fl;MC mammary tissues, respectively. Epithelial development was sparse and many epithelial cells failed to expand to accommodate functional alveoli. (D) Section from an α-catenin control mouse (α-catenin fl/+;WC) mammary tissue at the third parturition in which alveoli were expanded and milk and milk fat secretion was evident in the lumina. (E) Section from a mouse mammary gland at the third parturition in which α-catenin was inactivated by the WAP-Cre transgene (α-cat fl/fl;WC). Epithelial development appeared comparable to the first pregnancy shown in Fig. 2B. (F), Section from a mouse mammary gland at the third parturition in which α-catenin was inactivated by the MMTV-Cre transgene (α-cat fl/fl;MC). Epithelial development appeared comparable to the first pregnancy shown in Fig. 2C. ln, lymph node.
An evaluation of the ultrastructure of epithelial clusters using electron microscopy (Fig. 3) confirmed some of the features already evident at the level of light microscopy. At parturition, these epithelial clusters did not contain a central lumen and thus, did not display the usual organized orientation towards the lumen (Fig. 3B) as observed in the α-catenin fl/fl controls (Fig. 3A). The epithelial cells had large nuclei with little cytoplasm, and they displayed low to no secretory activity. However, myoepithelial cells were localized by immunohistochemistry at the periphery and were in contact with the basal lamina indicating a normal distribution as compared to the α-catenin fl/fl controls (data not shown).
Fig. 3.

Electron microscopic analysis of α-catenin fl/fl control and α-catenin deficient mammary tissue. (A) Micrograph of mammary tissue from α-catenin fl/fl control mice at parturition. Cells were stretched along the circumference of the lumen and appeared secretory as evidenced by milk fat droplets (arrow). (B) Micrograph of mammary tissue from a α-cat fl/fl;WC mouse at parturition. Note the clustering of cells and lack of a lumen (arrow) and secretory evidence. lu, lumen; s, secretory cell; nu, nucleus.
Evidence suggesting that α-catenin is an invasion suppressor (Van Aken et al., 2001) led us to investigate the consequences of α-catenin deletion over multiple pregnancies facilitating extensive deletion of the α-catenin gene through repeated activation of the WAP gene promoter and the MMTV-LTR. Loss of α-catenin over three pregnancies did not result in hyperproliferation of mammary epithelium or invasive characteristics (Fig. 2D-F). In fact, histological sections of mammary glands after three pregnancies appeared similar to those biopsied after the first pregnancy.
2.3. Alpha-catenin is required for alveolar differentiation and mammary function
Mammary functional output was established by weighing litters and analysis of milk gene expression by northern blot. Pups born to α-cat fl/fl;WC mothers were weighed on days one, five, ten and fifteen after parturition. The values were expressed as weight per pup as compared with α-cat fl/+;WC controls (Fig. 4A). The sizes of litters born to both groups of mothers were comparable in number and the pups did not display any growth defects in utero. However, by day five after parturition, it was evident that pups born to α-cat fl/fl;WC mothers failed to thrive as compared to the α-catenin fl/fl control litters. By day 15 after parturition the difference between the α-cat fl/fl;WC and α-catenin fl/fl control groups had become statistically significant (*p < 0:05). Throughout lactation, litters of α-catenin deficient dams remained approximately 50% of the weight of pups born to α-catenin fl/fl control dams. Litters which pups did not survive the lactation period were reduced in size to 50% by day 5 and to 25% by day 10 (*p < 0:1) (Fig. 4B).
Fig. 4.
Analysis of mammary output: Litter weights and northern analysis of milk protein gene expression in α-catenin deficient mammary tissue. (A) Weight of pups born to mothers with α-catenin deficient mammary glands. Litters (n = 4) that survived during the lactation period were weighed on days 1, 5, 10, and 15 after birth. Data represents the mean ± SEM; *p < 0.05. (B) Litters (n = 4) that failed to survive were counted on days 1, 5, and 10. Data represents the mean ± SEM; *p < 0.1. Open bars denote litters born to α-cat fl/+;WC mothers and hatched bars denote litters born to α-cat fl/fl;WC mothers. (C) Northern analysis of β-casein, WAP, WDNM1, K18, and GAPDH mRNA at parturition of indicated genotypes, D MMTV-Cre D.
To evaluate the differentiation status of mammary epithelium at parturition, the presence of milk protein mRNA was established by northern blot analyses (Fig. 4C). Both WAP and β-casein mRNA levels were reduced in a-cat fl/fl;WC and α-cat fl/fl;MC dams. Although β-casein was detectable in α-cat fl/fl;MC tissue, WAP was undetectable. Expression of WDNM1 was only reduced in the α-cat fl/fl;MC dams. Keratin 18 mRNA, which is normally used as an indicator of epithelial content, appeared to be upregulated or enriched in α-cat fl/fl;WC and α-cat fl/fl;MC tissues. Since the upregulation was less pronounced in α-cat fl/fl;MC tissue it was probably not the result of an enrichment of non-milk genes. The expression of WDNM1, which is a marker for cellular differentiation during early pregnancy (Robinson et al., 1995), demonstrated that the initial developmental phase occurred normally in α-cat fl/fl;WC, but was compromised in α-cat fl/fl;MC mammary tissue. These results demonstrate that α-catenin-deficient mammary epithelium was impaired in producing normal amounts of milk proteins and thus provides an initial molecular explanation for the failure of these mice to nurse their offspring. Furthermore, these data emphasize the importance of α-catenin in the functional differentiation of alveolar epithelial cells.
To further understand the functional status of α-catenin-null epithelium at parturition, we examined the expression of functional marker proteins preferentially expressed in secretory epithelial cells (Fig. 5). The Na–K–Cl co-transporter, NKCC1 (Shillingford et al., 2002), was present at high levels on the basolateral membranes of mammary epithelial cells from the α-catenin fl/fl controls (Fig. 5A, yellow arrow). In contrast, NKCC1 was not present in epithelial clusters of α-cat fl/fl;WC or α-cat fl/fl;MC tissues (Fig. 5B and C, white arrows) indicating a loss of cell identity. In epithelial clusters that had failed to expand into functional alveoli, α-catenin was not detected (Fig. 5E and F, white arrows). Occasional NKCC1 localization in these cells was no longer located at the basolateral membrane demonstrating a loss of polarity. Thus, loss of α-catenin coincides with loss of NKCC1. A distinct advantage of the mosaic expression of the WC and MC transgenes was the presence of expanded alveoli that expressed NKCC1 and α-catenin normally (Fig. 5C and F, yellow arrow). Alpha-catenin deficient epithelial clusters also failed to express a Na–Pi co-transporter (Npt2b) (Fig. 5G-I) and WAP (Fig. 5Jn-L, white arrows). Npt2b is normally expressed on the luminal surface of epithelial cells and is a hallmark for functional secretion (Shillingford et al., 2002), and WAP is normally found at the luminal surface of secretory epithelial cells, as well as in the lumen itself (yellow arrows) (Burdon et al., 1991).
Fig. 5.

Expression of differentiation markers in conditionally deleted α-catenin mammary tissues. Immunofluorescence labeling of α-catenin fl/fl control and conditionally null mammary tissues was performed and all tissues were counterstained with DAPI for nuclear localization. (A–C) E-cadherin (green) and NKCC1 (red) were co-expressed (yellow arrows) in α-catenin fl/fl control tissue in which the floxed α-catenin gene was not deleted with Cre recombinase (A). Loss of NKCC1 (white arrows) was seen in epithelial cell clusters which had failed to expand in α-cat fl/fl;WC (B) and α-cat fl/fl;MC (C) glands. (D–F) E-cadherin (green) and α-catenin (red) were co-expressed (yellow arrow) in α-catenin fl/fl control tissue in which the floxed α-catenin gene was not deleted with Cre recombinase (D). Loss of α-catenin (white arrows) was evident in epithelial cells, which had failed to expand in α-cat fl/fl;WC and α-cat fl/fl;MC (E and F, respectively) glands but was normal in neighboring alveoli (yellow arrows). (G–I) E-cadherin (green) was expressed at cellular junctions and Npt2b (red) was expressed on the luminal epithelial surface of expanded alveoli (yellow arrow) in α-catenin fl/fl control tissue in which the floxed α-catenin gene was not deleted with Cre recombinase (G). Loss of Npt2b (white arrows) was apparent in epithelial cells, which had failed to expand in α-cat fl/fl;WC and α-cat fl/fl;MC glands (H and I, respectively), but was normal in neighboring normal alveolar structures (yellow arrows). (J–L) E-cadherin (green) was expressed at cellular junctions and WAP (red) was expressed in the lumen of expanded alveoli (yellow arrow) in α-catenin fl/fl control tissue in which the floxed α-catenin gene was not deleted with Cre recombinase (J). Loss of WAP (white arrows) was evident in alveolar structures that had failed to expand in α-cat fl/fl;WC and α-cat fl/fl;MC (K and L, respectively), but was normal in neighboring alveolar structures (yellow arrows). (M–O) E-cadherin (green) was expressed at cellular junctions and Stat5a (red) was present in the nucleus of epithelial cells of expanded alveoli (yellow arrow) in α-catenin fl/fl control tissue in which the floxed α-catenin gene was not deleted with Cre recombinase (M). Epithelial cell clusters, as well as expanded alveoli of α-cat fl/fl;WC and α-cat fl/fl;MC (N and O, respectively) displayed Stat5a in the nucleus (yellow arrows). Although Stat5a was sometimes absent in clusters (O, white arrow).
Lactation is controlled by systemic lactogenic hormones, most prominently prolactin that activates the transcription factor Stat5a (Liu et al., 1996, 1997). To establish whether α-catenin-null cells can respond to prolactin signaling, we examined the localization of Stat5a in these tissues at parturition. In α-catenin fl/fl epithelium, nuclear Stat5a co-localization with DAPI was observed (Fig. 5M, yellow arrow). In the epithelial clusters from α-cat fl/fl;WC and α-cat fl/fl;MC mammary tissue nuclear Stat5a was observed variably (Fig. 5N and O, yellow and white arrows), suggesting that the clustered epithelial cells can partially respond to prolactin. Assessing all the markers tested, it was evident that epithelial cells lacking α-catenin are partially responsive to prolactin but are nevertheless impaired in functional differentiation.
2.4. Alpha-catenin is required for cell survival
Impaired epithelial clusters of α-cat fl/fl;WC and α-cat fl/fl;MC mammary tissue had failed to display proteins that indicate functional differentiation. To establish whether this resulted in reduced cell survival, the level of apoptosis was examined. The number of Apoptag positive cells was determined for α-catenin fl/fl controls, α-cat fl/fl;WC and α-cat fl/fl;MC at parturition. The percentages obtained were 0.2% for α-catenin fl/fl controls, 7.6% for α-cat fl/fl;WC and 2.5% for α-cat fl/fl;MC. Statistical analysis demonstrated that α-cat fl/fl;WC and α-cat fl/fl;MC results were significantly different from α-catenin fl/fl controls, but not statistically significant from each other (*p < 0:05) (Fig. 6). Thus, the loss of α-catenin in mammary epithelium results in increased apoptosis, which is in contrast to the loss of α-catenin in skin that resulted in hyperproliferation.
Fig. 6.

Apoptosis in α-catenin deficient tissues. Apoptag staining was performed at d 1 of lactation. In α-cat fl/fl;WC and α-cat fl/fl;MC tissues, apoptosis was significantly increased compared to α-catenin fl/fl control tissues. Data represents the mean ± SEM; *p < 0.05.
3. Discussion
This study demonstrates that the biogenesis of a functional mammary gland depends on the presence of α-catenin, and that its loss does not result in the hyperproliferation of mammary epithelium. At parturition, loss of α-catenin coincided with disorganized and non-polarized mammary epithelial cell clusters. It is not known whether these cells were organized at an earlier point in development and lost this feature or whether they never achieved organization during mammopoiesis. In addition, the absence of secretory markers, such as Npt2b and WAP, demonstrates that these tissues had failed to correctly respond to pregnancy-mediated signals emphasizing the importance of α-catenin in functional differentiation. However, the presence of the prolactin dependent transcription factor Stat5a in the nucleus of cells lacking α-catenin indicates that that PrlR was present and functional, at least to some extent, on the surface of these cells. Thus, these data suggest that epithelial clusters devoid of α-catenin may be partially differentiated, and thus indicating a clear necessity for α-catenin to reach a state of functional differentiation. This was supported at the molecular level with α-catenin deficient tissues that exhibited a reduction in milk protein gene expression, namely β-casein, WAP, and WDNM1.
Apoptosis analysis of mammary cells in which α-catenin was conditionally inactivated indicated that these cells were in the process of undergoing programmed cell death. In mice whose litters died by days 5–10, epithelial cells resembled cells from involuted mammary tissue (data not shown). It is likely that α-catenin is a critical component when the secretory epithelium is at its maximum challenge during lactation. Thus, these data imply that without the structural support of α-catenin, mammary epithelial cells proceed toward an apoptotic pathway. The relationship between structural molecules and apoptosis was also observed in the conditional deletion of E-cadherin in the mammary gland. In these mice, mammary epithelial cells involute shortly after parturition and mutant glands are compromised in their ability to produce milk and support litters (Boussadia et al., 2002).
In carcinomas, it has been reported that α-catenin is reduced and/or absent suggesting that these molecules may be tumor suppressors (Van Aken et al., 2001). In skin, this hypothesis was confirmed through the conditional inactivation of the α-catenin gene, which resulted in the hyperproliferation of keratinocytes (Vasioukhin et al., 2001). In the mammary gland, hyperproliferation was not detected in the absence of α-catenin, even after several cycles of Cre activation, suggesting that in mammary epithelial cells it was not a bona fide tumor suppressor. Similar to α-catenin deficient mammary tissue, tumors were not detected in mutant glands lacking E-cadherin (Boussadia et al., 2002). The lack of tumor development in both α-catenin and E-cadherin deficient glands was not expected given that both are putative tumor suppressor molecules in vitro and in vivo (Meiners et al., 1998; Van Aken et al., 2001). The high level of apoptosis in these glands may contribute to the lack of hyperplasias or tumors in α-cat fl/fl;WC and α-cat fl/fl;MC mice after multiple pregnancies. This finding is important because it contrasts the numerous reports implicating α-catenin as a tumor suppressor. It can, however, not be excluded that loss of α-catenin contributes to tumor progression.
4. Experimental Procedures
4.1. Mice
Mammary tissue of C57Bl/6 females at various stages of development was analyzed for the presence of α-catenin. Stages analyzed include the virgin mammary fat pad devoid of epithelium (3 weeks), mature virgin (nulliparous; 10 weeks), gestation (days 11, 14, and 18 of pregnancy), lactation (days 1 and 10) and involution (days 2 and 10). Gestation days were determined by plug checking and noting the plug date as day 1.
Mice carrying floxed α-catenin genes (Vasioukhin et al., 2001) were mated to mice carrying the WAP-Cre (WC) and MMTV-Cre D (MC) transgenes (Wagner et al., 1997). The WC and MC transgenes were identified by PCR as described previously (Wagner et al., 1997). The presence of the α-catenin floxed, wild-type or null alleles were identified by PCR using the following forward and reverse primers 5′-CAT TTC TGT CAC CCC CAA AGA CAC-3′ and 5′-GCA AAA TGA TCC AGC GTC CTG GG-3′, respectively. Resulting females, which carried both the WAP-Cre or MMTV-Cre transgenes and the double floxed α-catenin gene were analyzed for mammary tissue development. All animals used in the described studies were treated in accordance with Public Health Service Policies and Federal Regulations.
4.2. Histological evaluation of mammary tissue
The inguinal mammary gland (number four) was biopsied at the indicated times of development and fixed for 4–12 h in Tellyesniczky's fixative, paraffin-embedded, sectioned 5 μm thick, and stained with hematoxylin and eosin.
4.3. Assay of mammary function through pup weights
Litters born to indicated transgenic females were weighed to assess mammary output and function. Litters (n = 4) were weighed on days one, five, ten, and fifteen. An average of four litters was compared by Student's t-Test analysis.
4.4. RNA blot hybridization
Total cellular RNA was purified from mammary tissue of indicated mice at parturition and tested for β-casein, WAP, WDNM1, and keratin 18 as previously described (Robinson et al., 1995). After hybridization, membranes were washed in 2 × SSC + 0.1% SDS (1 × SSC is 0.15 M NaCl plus 0.015 M sodium citrate) twice for 15 min per wash and 1 time with 1 × SSC + 0.1%SDS for 15 min. The signals were visualized with a 6-h exposure using Kodak Biomax-AR film.
4.5. Western blot analysis
Protein was isolated from mammary tissue as described (Liu et al., 1996). A total of 40 μg of protein was separated by electrophoresis in 7% Tris-acetate gels (Invitrogen, Carlsbad, CA) and transferred to PVDF membranes (Invitrogen, Carlsbad, CA) using a Novex Xcell II Mini-Cell and Blot Module (Invitrogen, Carlsbad, CA). Membranes were blocked for 30 min at room temperature in TBST (Tris-buffered saline, pH 7.4, with 0.05%Tween 20) containing 3% (wt/vol) nonfat dried milk and probed with rabbit polyclonal anti-alpha-catenin at 1:10,000 dilution (Sigma Chemical Co., St. Louis, MO) and mouse monoclonal anti-E-cadherin 1:2500 (BD-Transduction Laboratories, Lexington, KY) for 1 h at room temperature. Subsequently, membranes were incubated with an HRP-linked anti-mouse or anti-rabbit secondary antibody (BD-Transduction Laboratories, Lexington, KY) for 30 min at room temperature. Specifically bound antibodies were visualized by using SuperSignal West Pico Chemiluminescent Substrate according to manufacturer's instructions (Pierce Biotechnology, Rockford, IL).
4.6. Immunohistochemistry
Mammary tissues were fixed in Tellyesniczky's fixative for 4–12 h at room temperature and embedded in paraffin and sectioned at 5 μm. Sections were cleared in xylene and rehydrated. Antigen retrieval was performed by heat treatment using an antigen unmasking solution (Vector Laboratories, Burlingame, CA) and tissue sections were blocked for 30 min in 3% goat or horse serum/PBS. Sections were incubated with the following primary antibodies at 37 °C for one h: mouse monoclonal anti-E-cadherin at 1:100 (BD-Transduction Laboratories, Lexington, KY); rabbit polyclonal anti-alpha-catenin at 1:500 (Sigma Chemical Co., St. Louis, MO). rabbit polyclonal anti-Na–K–Cl cotransporter 1 (NKCC1) at 1:1000 and rabbit polyclonal anti-Npt2b (Npt2b_Na–Pi cotransporter type II b) at 1:100 were the kind gifts from Jim Turner, National Institute of Dental and Craniofacial Research, National Institutes of Health and Jurg Biber, Department of Physiology, University of Zurich, Switzerland, respectively. Both rabbit polyclonal anti-Stat5a (Stat, signal transducer and activator of transcription) at 1:200 (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti-WAP at 1:600 (Burdon et al., 1991) were incubated at 4 °C overnight. Tissues were incubated in secondary antibodies FITC-conjugated anti-mouse (Alexafluor 488) or Texas Red-conjugated anti-rabbit (Alexafluor 594) used at 1:400 (Molecular Probes, Eugene, OR) for 30 min in the dark at room temperature. Sections were mounted in Vectashield mounting medium for fluorescence with DAPI (Vector Laboratories, Inc., Burlingame, CA). Fluorescence was visualized with an Olympus BX51 microscope equipped with FITC, Texas Red, and DAPI filters. Images were captured and processed using a Nikon DXM 1200 digital camera and Nikon Act-1 Version 2.12 Software, respectively (Nikon, Japan).
4.7. Apoptosis assay
For apoptosis assays, Apoptag Red In Situ Apoptosis Detection Kit was used according to manufacturer's instructions and counterstained with DAPI (Intergen Co., Purchase, NY). Each apoptosis sample counted represents a minimum of five random fields (at X400 magnification) and a minimum of 1000 total cells per tissue section for each mouse. A minimum of three mice per genotype were collected and analyzed. We were able to use a Student's t-Test analysis for the statistical analysis of Apoptag positive cells with the data sets that included three mice each contributing 1000 individual units (cells counted), and a total of over 3000 cells counted for each genotype.
4.8. Electron microscopy
Electron microscopic analysis of mammary tissue was described previously (Miyoshi et al., 2002). Tissues were fixed in 3.8% paraformaldehyde and 0.025% glutaraldehyde in PBS. After postfixation in 2% osmium tetroxide in 0.5 M sodium cacodylate buffer they were dehydrated and embedded in plastic. Ultrathin sections were contrasted with Karnovsky's lead hydrate and uranyl acetate and examined with a JEOL 1010 electron microscope.
Acknowledgements
We are grateful to Dr. Gertraud Robinson for her experimental and scholarly advice throughout the course of this work.
References
- Beavon IR. The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation. Eur. J. Cancer. 2000;36:1607–1620. doi: 10.1016/s0959-8049(00)00158-1. [DOI] [PubMed] [Google Scholar]
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev. 2002;115:53–62. doi: 10.1016/s0925-4773(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Bukholm IK, Nesland JM, Karesen R, Jacobsen U, Borresen-Dale AL. E-cadherin and alpha-, beta-, and gamma-catenin protein expression in relation to metastasis in human breast carcinoma. J. Pathol. 1998;185:262–266. doi: 10.1002/(SICI)1096-9896(199807)185:3<262::AID-PATH97>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bukholm IK, Nesland JM, Borresen-Dale AL. Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients (see comments) J. Pathol. 2000;190:15–19. doi: 10.1002/(SICI)1096-9896(200001)190:1<15::AID-PATH489>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Burdon T, Wall RJ, Shamay A, Smith GH, Hennighausen L. Over-expression of an endogenous milk protein gene in transgenic mice is associated with impaired mammary alveolar development and a milchlos phenotype. Mech. Dev. 1991;36:67–74. doi: 10.1016/0925-4773(91)90073-f. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 1998;12:449–455. doi: 10.1101/gad.12.4.449. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev. Cell. 2001;1:467–475. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Hennighausen L. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol. Endocrinol. 1996;10:1496–1506. doi: 10.1210/mend.10.12.8961260. [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Meiners S, Brinkmann V, Naundorf H, Birchmeier W. Role of morphogenetic factors in metastasis of mammary carcinoma cells. Oncogene. 1998;16:9–20. doi: 10.1038/sj.onc.1201486. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Meyer B, Gruss P, Cui Y, Renou JP, Morgan FV, et al. Mammary epithelial cells are not able to undergo pregnancy-dependent differentiation in the absence of the helix-loop-helix inhibitor Id2. Mol. Endocrinol. 2002;16:2892–2901. doi: 10.1210/me.2002-0128. [DOI] [PubMed] [Google Scholar]
- Nanba D, Nakanishi Y, Hieda Y. Changes in adhesive properties of epithelial cells during early morphogenesis of the mammary gland. Dev. Growth Differ. 2001;43:535–544. doi: 10.1046/j.1440-169x.2001.00596.x. [DOI] [PubMed] [Google Scholar]
- Neville MC. Physiology of lactation. Clin. Perinatol. 1999;26:251–279. [PubMed] [Google Scholar]
- Robinson GW, McKnight RA, Smith GH, Hennighausen L. Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development. 1995;121:2079–2090. doi: 10.1242/dev.121.7.2079. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Miyoshi K, Flagella M, Shull GE, Hennighausen L. Mouse mammary epithelial cells express the Na–K–Cl cotransporter, NKCC1: characterization, localization, and involvement in ductal development and morphogenesis. Mol. Endocrinol. 2002;16:1309–1321. doi: 10.1210/mend.16.6.0857. [DOI] [PubMed] [Google Scholar]
- Torres M, Stoykova A, Huber O, Chowdhury K, Bonaldo P, Mansouri A, et al. An alpha-E-catenin gene trap mutation defines its function in preimplantation development. Proc. Natl Acad. Sci. USA. 1997;94:901–906. doi: 10.1073/pnas.94.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aken E, De Wever O, Correia da Rocha AS, Mareel M. Defective E-cadherin/catenin complexes in human cancer. Virchows Arch. 2001;439:725–751. doi: 10.1007/s004280100516. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, et al. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 2001;10:545–553. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]