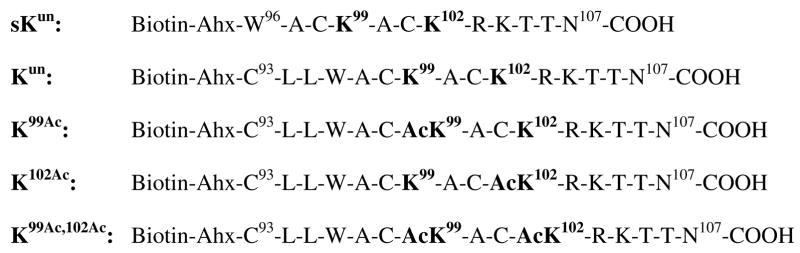Abstract
The recruitment of the bromodomains of CREB-binding protein (CBP) and p300 by the acetylated myogenic transcription factor MyoD was previously shown to be critical for the enhanced MyoD transcriptional activity following acetylation at its Lys99 and Lys102 positions. However, the modes of binding interactions of the bromodomains of CBP and p300 with acetylated MyoD have not been well-characterized. In the current study, by employing a panel of MyoD peptides encompassing the 99 and 102 positions, we showed that Lys99 monoacetylation and Lys99/Lys102 double acetylation defined the critical binding interfaces with the bromodomains of CBP and p300, respectively. This also represented the first identification of a recognition motif for the p300 bromodomain and revelation of the differential recognition motifs for the bromodomains of CBP and p300. This information could be exploited for developing novel tools for structural and functional studies of the highly homologous CBP and p300 transcriptional coactivators.
Keywords: Acetylated MyoD, Bromodomain, CBP, p300, Binding
Reversible lysine Nε-acetylation on proteins has been increasingly recognized to play critical roles in regulating multiple pivotal cellular processes such as gene transcription, cell-cycle progression, and metabolism [1–6]. This functional significance could be derived from the reversible lysine acetylation itself which is coordinated by protein acetyltransferases and protein deacetylases, as well as the recruitment of bromodomain-containing proteins following lysine acetylation. Bromodomain represents a family of acetyl-lysine binding protein modules that contain ~110 amino acid residues and are present in many chromatin-associated proteins including transcription coactivators (e.g. CBP, p300, PCAF, and GCN5), transcription factor TAFII250, the BET family of nuclear proteins (e.g. Brd2, Brd4, and Bdf1), and ATP-dependent chromatin-remodeling complexes (e.g. SWI/SNF) [7,8]. Lysine acetylation was first observed on histone proteins, but an increasing list of non-histone proteins, such as various transcription factors, HIV Tat protein, alpha-tubulin, and acetyl-coenzyme A synthetases, were also found to be acetylated [1–6].
MyoD is a muscle-specific transcription factor essential for muscle cell terminal differentiation and muscle cell programming [9]. Its transcriptional activity has been demonstrated to be enhanced by specific lysine acetylation at the 99, 102, and 104 positions, catalyzed by the acetyltransferase activity of transcriptional coactivators CBP, p300, and PCAF [10–13]. It has also been shown that the recruitment of the bromodomains of CBP and p300 by acetylated MyoD is essential for the observed enhanced transcriptional activity following acetylation at the Lys99 and Lys102 positions of MyoD [14]. However, the modes of binding interactions of the bromodomains of CBP and p300 with acetylated MyoD have not been well-characterized. Even though CBP and p300 are highly homologous to each other with 91% sequence identity for human proteins, and were initially suggested to be functionally equivalent [15–17], their differential functional roles have been demonstrated in different cellular processes [18–21]. Furthermore, while the CBP bromodomain was shown to be able to bind to a Lys382 monoacetylated peptide derived from the human tumor suppressor protein p53, the p300 bromodomain was unable to do so [8,22]. These previously documented differences between the bromodomains of CBP and p300 and between these two proteins themselves further prompted us to examine the possible differential modes of binding interactions between acetylated MyoD and the bromodomains of CBP and p300.
In the current study, using a panel of MyoD peptides encompassing the 99 and 102 positions (Fig. 1), glutathione-S-transferase (GST)-fused bromodomains of CBP and p300, and an in vitro peptide–bromodomain binding assay, we have examined the individual and collective contributions of acetylation at the Lys99 and Lys102 positions to the binding interactions with the bromodomains of CBP and p300.
Fig. 1.
Biotinylated MyoD peptides used in the current study. AcK, Nε-acetyl-lysine; Ahx, ε-amino-caproic acid.
Materials and methods
Peptide synthesis and purification
All peptides were synthesized on a PS3 peptide synthesizer (Protein Technologies Inc., Tucson, AZ, USA) based on the Fmoc chemistry strategy [23]. Fmoc-ε-amino-caproic acid and all the other Fmoc-protected amino acids and resins were purchased from Novabiochem. While four equivalents of Fmoc-protected amino acids were used in 1-h coupling reactions, 2 equivalents of Biotin-NHS (Calbiochem) were used in 2-h double coupling reactions to couple biotin to the N-terminal ε-amino-caproyl in each peptide. All the peptides were cleaved from the resin by reagent K (83.6% (v/v) trifluoroacetic acid, 5.9% (v/v) phenol, 4.2% (v/v) double deionized water (ddH2O), 4.2% (v/v) thioanisole, 2.1% (v/v) ethanedithiol), precipitated in cold diethyl ether, and purified by reversed-phase high pressure liquid chromatography (HPLC) on a preparative C18 column (100 Å, 2.14 × 25 cm), eluting with a gradient of ddH2O containing 0.05% (v/v) of trifluoroacetic acid and acetonitrile containing 0.05% (v/v) of trifluoroacetic acid. The pooled HPLC fractions were stripped of acetonitrile and lyophilized to give all peptides as puffy white solids. Peptide purity (>95%) was verified by reversed-phase HPLC on an analytical C18 column (100 Å, 0.46 × 25 cm), and their molecular weights were confirmed by either matrix assisted laser desorption ionization-time of flight (MALDI-TOF) or electrospray ionization (ESI) mass spectrometric analysis.
Expression and purification of GST-CBP (1082–1197) and GST-p300 (1047–1157)
Following the transformation of pGEX4T-3·CBP (1082–1197) (derived by subcloning from pGEX2T·CBP (1079–1457) which is a kind gift from Prof. Annick Harel-Bellan) or pGEX2TKx·p300 (1047–1157) (a kind gift from Prof. W. Lee Kraus) that define, respectively, the human CBP bromodomain [22,24] or the human p300 bromodomain [24,25] into the Escherichia coli strain BL21-CodonPlus (DE3)-RIL, one of the resulting colonies was used to inoculate a 50-mL Luria Broth containing 100 μg/mL of ampicillin, and the culture was grown at 37 °C for 20 h. This culture was subsequently used to inoculate a 450-mL Luria Broth also containing 100 μg/mL of ampicillin, and the culture was grown at 37 °C until the optical density (OD) at 600 nm reached 0.8–1.0. Fresh 1.0M isopropyl-1-thio-β-D-galactopyranoside (IPTG) was then added to the culture to a final concentration of 0.1 mM, and the culture was grown at 25 °C for an additional 20 h (or at 30 °C for an additional 1 h for the expression of GST-p300 (1047–1157)). The cells were harvested by centrifugation at 6000 rpm and 4 °C for 10 min, and the pellet was resuspended in 10 mL of the cold lysis buffer (10 mM Tris·HCl (pH 7.5), 800 mM NaCl, 2 mM EDTA, 0.8% (v/v) Triton X-100, 10% (v/v) glycerol). Resuspended cells were lysed by double passage through a French pressure cell at 700 psi and centrifuged at 12,000 rpm and 4 °C for 30 min to remove cell debris. The supernatant was incubated with rotation on a Nutator at 4 °C for 4 h with 35 mg of the glutathione-agarose (that had been pre-swollen in 10 mL of ddH2O, equilibrated with 10 mL of the lysis buffer, and pelleted by centrifugation at 1400 rpm and 4 °C for 20 s with the supernatant being discarded). This was followed by centrifugation at 1400 rpm and 4 °C for 20 s. The pellet was washed four times, each time with 10 mL of the lysis buffer, and centrifuged at 1400 rpm and 4 °C for 20 s with the supernatant being discarded. The GST fusion protein was eluted off of the washed pellet with 4 × 1 mL of the elution buffer containing 5 mM glutathione in the lysis buffer (pH 7.5). The pooled fractions were dialyzed against the peptide–bromodomain binding assay buffer (50 mM Tris·HCl (pH7.5), 50 mM NaCl), concentrated by ultrafiltration, and used in the peptide–bromodomain binding assay (vide infra). The protein concentration was determined by the Bradford assay.
In vitro peptide–bromodomain binding assay
Part of the following assay conditions was derived from Ref. [22]. A homogeneous mixture consisting of 16 μL of GST-CBP (1082–1197) (or GST-p300 (1047–1157)) (25 μM in binding buffer), 4 μL of immunoglobulin G (IgG) (SIGMA (catalog # M5534), in 10 mM PBS (pH 7.4), 1% BSA, and 0.1% sodium azide), and 20 μL of a MyoD peptide (200 μM in binding buffer) was incubated with rotation on a Nutator at room temperature for 1 h. At the same time, an agarose mixture was prepared by mixing streptavidin-agarose (50% slurry from Novagen, catalog # 69203-3) and protein A-agarose (50% slurry from Calbiochem, catalog # IP02) at a ratio of 8:1 (v/v), followed by washing with the binding buffer (twice, each with a bed volume of the agarose). 90 μL of a 50% slurry (in binding buffer) of thus prepared agarose mixture was then added to the above homogenous mixture of a GST-bromodomain, aMyoD peptide, and IgG. The resulting mixture was incubated with rotation at room temperature for an additional 45 min. After centrifugation at 3000 rpm and 4 °C for 5 min, the pellet was washed with 3× 200 μL of the wash buffer containing 50 mM Tris·HCl (pH 7.5), 0.9M (or 1.2 M) KCl, 0.1% (v/v) NP-40, and centrifuged at 3000 rpm and 4 °C for 5 min, with the supernatant being discarded. Of note, 1.2M was also the highest KCl concentration used by Polesskaya et al. in their comparative assessment of the binding interactions of acetylated and non-acetylated MyoD proteins with the bromodomain of CBP [14]. To the washed pellet was added 40 μL of a 1× SDS gel-loading buffer. The mixture was vortexed vigorously, boiled for 10 min, centrifuged, and 30 μL of it was loaded onto a 10% SDS–PAGE gel. The bands for IgG and GST-bromodomain that were retained on the agarose mixture were visualized with “blue silver” staining [26]. Briefly, after electrophoresis, a protein gel was treated with a destaining solution (50% (v/v) methanol, 10% (v/v) glacial acetic acid, and 40% (v/v) ddH2O) for 2 h before the gel was treated overnight with the colloidal “blue silver” aqueous solution that contained 1.2% Coomassie Blue G-250, 10% (NH4)2SO4, 10% H3PO4, and 20% methanol. The stained gel was then washed with ddH2O, dried, and subjected to densitometry analysis to determine the adjusted band intensities for IgG and GST-bromodomain.
Densitometry analysis
The ImageJ software (version 1.37) available from U.S. National Institutes of Health was used [27]. Briefly, a dried SDS–PAGE gel was scanned and saved as a JPEG image, and the adjusted band intensities for IgG and GST-bromodomain were determined.
Results and discussion
Design of MyoD peptides
Fig. 1 shows the MyoD peptides used in the current study. All peptides were biotinylated for use in the in vitro peptide–bromodomain binding assay (vide supra). The sequence (amino acid residues 93–107) of human MyoD [28] that is located between the basic helix–loop–helix (bHLH) domain and the cysteine–histidine (C/H) rich region was used as a template for constructing the following MyoD peptides (“un” stands for “unacetylated”, “Ac” stands for “acetylated”): Kun, an unacetylated negative control peptide; K99Ac, the peptide monoacetylated at Lys99; K102Ac, the peptide monoacetylated at Lys102; and K99Ac,102Ac, the peptide diacetylated at Lys99 and Lys102. In addition, sKun (“s” stands for “shorter”) that was based on a shorter MyoD sequence (96–107) was included in our study as a second unacetylated negative control peptide. Together with Kun, the inclusion of sKun in our study could help to ensure the maximal removal of non-specific binding interaction between a peptide and a bromodomain.
In vitro peptide–bromodomain binding assay
Fig. 2 shows a schematic diagram for the in vitro binding assay that was used in the current study to quantify the binding interaction between a MyoD peptide and a bromodomain. This binding assay was a derivative version of that originally developed by Mujtaba et al. [22], with the following two modifications. First, as compared to the original assay format, we included IgG to be incubated with a GST-bromodomain and a biotinylated peptide at the first step of the assay, and used a mixture of protein A-agarose and streptavidin-agarose, instead of streptavidin-agarose only, to capture simultaneously biotinylated species and IgG from the incubation mixture. It is worth noting that inclusion of protein A-agarose and IgG had no interference with the bromodomain-peptide binding (unpublished observation). Second, as compared to the original assay format, instead of using Western blotting with anti-GST antibody to detect and quantify the peptide-bound GST-bromodomain at the last step of the assay, we used an improved Coomassie blue staining (“blue silver” staining [26]), which is easier and quicker than the Western blotting approach. One unique feature of the in vitro binding assay used in the current study is that the possible amount variation for the IgG protein retained on the protein A-agarose beads would reflect a parallel amount variation for the streptavidin-agarose beads remaining in tubes following repetitive manipulations during washing. Therefore, the amount of the peptide-bound GST-bromodomain could be normalized against the amount of IgG following the densitometry analysis of a single SDS–PAGE gel, affording the true relative binding affinities of a set of peptides for a single bromodomain.
Fig. 2.

A schematic diagram for the in vitro peptide–bromodomain binding assay used in the current study.
Acetylated MyoD peptides differentially bind to the bromodomains of CBP and p300
Each of the MyoD peptides shown in Fig. 1 was initially evaluated for its binding interaction with the GST-bromodomain of CBP (i.e. GST-CBP (1082–1197), see Materials and methods). As shown in Fig. 3, when a wash buffer containing 0.9 M of KCl was used, peptides K99Ac and K102Ac were observed to have comparable binding affinities for GST-CBP (1082–1197), but a slightly lower binding affinity was observed for peptide K99Ac,102Ac. Peptide Kun was observed to have a binding affinity that was twice that of peptide sKun. When the KCl concentration in a wash buffer was raised to 1.2 M, comparable binding affinities were observed for the following four peptides, i.e. sKun, Kun, K102Ac, and K99Ac,102Ac. However, their binding affinities were all lower than that of peptide K99Ac. The observed change in the relative binding affinities (i.e. from twofold difference to comparable) of the two negative control peptides (i.e. sKun and Kun) when the KCl concentration was raised from 0.9 Mto 1.2 Margued that non-specific binding interactions between peptides and GST-CBP (1082–1197) had been reduced to a minimum. Therefore, the relative binding affinities of acetylated peptides observed under 1.2 M KCl wash condition, rather than those observed under 0.9 M KCl wash condition, should reflect their relative extents of specific binding to GST-CBP (1082–1197).
Fig. 3.
SDS–PAGE and densitometry analyses indicating the relative binding affinities of MyoD peptides for the bromodomains of CBP and p300 under two different salt washing conditions. A bar graph based on densitometry analysis is shown below each representative protein gel image. GST-Brd band intensities were normalized based on band intensities for the internal control IgG. Each bar in a bar graph represents the average GST-Brd band intensity measured for a duplicate sample within each single binding experiment. Bar sKun represents the average intensity for the GST-Brd bands in lanes 1 and 2 (peptide sKun was used in the experiment), bar Kun for the GST-Brd bands in lanes 3 and 4 (peptide Kun was used in the experiment), bar K99Ac for the GST-Brd bands in lanes 5 and 6 (peptide K99Ac was used in the experiment), bar K102Ac for the GST-Brd bands in lanes 7 and 8 (peptide K102Ac was used in the experiment), and bar K99Ac,102Ac for the GST-Brd bands in lanes 9 and 10 (peptide K99Ac,102Ac was used in the experiment). The same binding experiment was repeated two more times, and a pattern of GST-Brd band intensities consistent with that shown in this figure was observed. Exactly the same assay conditions were employed to assess the binding interactions between a GST-Brd (of CBP or p300) and peptides shown in Fig. 1.
Our observations thus suggested that (i) acetylation at Lys99 plays a more significant role than that at Lys102 in recruiting the CBP bromodomain, which is consistent with a previous observation by Polesskaya et al. [14] in which MyoD protein and its mutants were used instead of MyoD peptides and (ii) double acetylation at Lys99 and Lys102 attenuates the effect of monoacetylation at Lys99.
Following the binding experiments with CBP bromodomain, each of the MyoD peptides shown in Fig. 1 was further evaluated for its binding interaction with the GST-bromodomain of p300 (i.e. GST-p300 (1047–1157), see Materials and methods). As shown in Fig. 3, when a wash buffer containing 0.9 M of KCl was used, peptide K99Ac,102Ac was observed to have a higher binding affinity for GST-p300 (1047–1157) than both peptides K99Ac and K102Ac. When the KCl concentration in a wash buffer was raised to 1.2 M, a nearly same pattern of peptide binding to GST-p300 (1047–1157) was observed. Again, non-specific binding interactions between peptides and GST-p300 (1047–1157) appeared to have been reduced to a minimum when KCl concentrations were raised from 0.9 to 1.2 M, as suggested by the observed relative binding affinities of the two negative controls (i.e. peptides sKun and Kun) that were approaching each other following [KCl] increase from 0.9 to 1.2 M.
These observations thus suggested that neither Lys99 monoacetylation nor Lys102 monoacetylation plays an important role in recruiting the p300 bromodomain, rather, it is the double acetylation at Lys99 and Lys102 that promotes the binding to and recruitment of the p300 bromodomain. This pattern of recognition between the p300 bromodomain and the acetylated MyoD peptides is in stark contrast with that between the CBP bromodomain and the same peptides. While there has been documented examination of the molecular determinants for the binding of the CBP bromodomain to an acetyl-lysine-containing target sequence, the corresponding examination for the p300 bromodomain has been lacking [7,8,22]. Our results reported here may thus further suggest that, as a general rule, p300 bromodomain is unable to bind to a target sequence with only one acetylated Lys residue, but it is able to bind to a target sequence with two or perhaps more acetylated Lys residues with appropriate relative positioning. If this is true, it could explain the incapability of p300 bromodomain to bind to a p53 peptide harboring a monoacetylation at Lys382 [8,22].
In summary, by employing a panel of MyoD peptides encompassing the 99 and 102 positions, we have shown that Lys99 monoacetylation and Lys99/Lys102 double acetylation define the critical binding interfaces with the bromodomains of CBP and p300 transcriptional coactivators, respectively. This is not only the first revelation of the differential recognition motifs for the bromodomains of CBP and p300, but also the very first identification of a recognition motif for the p300 bromodomain. Peptide K99Ac,102Ac could thus be exploited for structural studies with the p300 bromodomain which are currently un-explored but are expected to be able to further our understanding of how this important protein module found in the critical transcriptional coactivator p300 recognizes its target sequences.
Our findings in the current study could also be exploited for developing novel tools (e.g. compounds capable of selectively blocking the binding interactions of the bromodomains of CBP and p300 with their target motifs) for a comparative functional examination of the highly homologous CBP and p300 in various biological processes. In this regard, peptides K99Ac and K99Ac,102Ac as well as their derivatives replacing acetyl-lysine with a non-hydrolyzable analog, such as Nε-methanesulfonyl-lysine that we developed recently [29], could serve as the lead compounds.
Acknowledgments
We are grateful for financial support (to W. Zheng) from the James L. and Martha J. Foght Endowment, The University of Akron Research Foundation, and The University of Akron Faculty Research Fellowship. The financial support (to Z. Wang) from U.S. National Institutes of Health (CA127590) is also highly appreciated. We thank Prof. W. Lee Kraus (Cornell University, USA) for the GST-p300 (1047–1157) plasmid and assistance with its expression and purification, Prof. Annick Harel-Bellan (CNRS, France) for the GST-CBP (1079–1457) plasmid, and Prof. Chrys Wesdemiotis and his research group at The University of Akron for their assistance with the mass spectrometric analysis of the peptides used in the current study. We also thank Prof. Amy Milsted and Mr. Adam Underwood (University of Akron), Dr. Xiaodong Zhang and Dr. Jianshi Yu (Case Western Reserve University) for their assistance with the Western blotting at the early stage of the current study. Mr. David Fatkins is thanked for his assistance with the expression and purification of GST-p300 (1047–1157).
References
- 1.Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 2.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 3.Hodawadekar SC, Marmorstein R. Chemistry of acetyl transfer by histone modifying enzymes: structure, mechanism and implications for effector design. Oncogene. 2007;26:5528–5540. doi: 10.1038/sj.onc.1210619. [DOI] [PubMed] [Google Scholar]
- 4.Batta K, Das C, Gadad S, Shandilya J, Kundu TK. Reversible acetylation of non histone proteins: role in cellular function and disease. Subcell Biochem. 2007;41:193–212. [PubMed] [Google Scholar]
- 5.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 6.Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489–5504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- 7.Mujtaba S, Zeng L, Zhou MM. Structure and acetyl-lysine recognition of the bromodomain. Oncogene. 2007;26:5521–5527. doi: 10.1038/sj.onc.1210618. [DOI] [PubMed] [Google Scholar]
- 8.Yang XJ. Lysine acetylation and the bromodomain: a new partnership for signaling. Bioessays. 2004;26:1076–1087. doi: 10.1002/bies.20104. [DOI] [PubMed] [Google Scholar]
- 9.Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol. 2005;16:585–595. doi: 10.1016/j.semcdb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Polesskaya A, Duquet A, Naguibneva I, Weise C, Vervisch A, Bengal E, Hucho F, Robin P, Harel-Bellan A. CREB-binding protein/p300 activates MyoD by acetylation. J Biol Chem. 2000;275:34359–34364. doi: 10.1074/jbc.M003815200. [DOI] [PubMed] [Google Scholar]
- 11.Polesskaya A, Harel-Bellan A. Acetylation of MyoD by p300 requires more than its histone acetyltransferase domain. J Biol Chem. 2001;276:44502–44503. doi: 10.1074/jbc.M106501200. [DOI] [PubMed] [Google Scholar]
- 12.Duquet A, Polesskaya A, Cuvellier S, Ait-Si-Ali S, Hery P, Pritchard LL, Gerard M, Harel-Bellan A. Acetylation is important for MyoD function in adult mice. EMBO Rep. 2006;7:1140–1146. doi: 10.1038/sj.embor.7400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartorelli V, Puri PL, Hamamori Y, Ogryzko V, Chung G, Nakatani Y, Wang JY, Kedes L. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol Cell. 1999;4:725–734. doi: 10.1016/s1097-2765(00)80383-4. [DOI] [PubMed] [Google Scholar]
- 14.Polesskaya A, Naguibneva I, Duquet A, Bengal E, Robin P, Harel-Bellan A. Interaction between acetylated MyoD and the bromodomain of CBP and/or p300. Mol Cell Biol. 2001;21:5312–5320. doi: 10.1128/MCB.21.16.5312-5320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arany Z, Sellers WR, Livingston DM, Eckner R. E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell. 1994;77:799–800. doi: 10.1016/0092-8674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 16.Arany Z, Newsome D, Oldread E, Livingston DM, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- 17.Lundblad JR, Kwok RPS, Laurance ME, Harter ML, Goodman RH. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature. 1995;374:85–88. doi: 10.1038/374085a0. [DOI] [PubMed] [Google Scholar]
- 18.Fonte C, Trousson A, Grenier J, Schumacher M, Massaad C. Opposite effects of CBP and p300 in glucocorticoid signaling in astrocytes. J Steroid Biochem Mol Biol. 2007;104:220–227. doi: 10.1016/j.jsbmb.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Kasper LH, Fukuyama T, Biesen MA, Boussouar F, Tong C, de Pauw A, Murray PJ, van Deursen JM, Brindle PK. Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Mol Cell Biol. 2006;26:789–809. doi: 10.1128/MCB.26.3.789-809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24:3619–3631. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]
- 21.Kalkhoven E. CBP and p300: HATs for different occasions. Biochem Pharmacol. 2004;68:1145–1155. doi: 10.1016/j.bcp.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 22.Mujtaba S, He Y, Zeng L, Yan S, Plotnikova O, Sachchidanand, Sanchez R, Zeleznik-Le NJ, Ronai Z, Zhou MM. Structural mechanism of the bromodomain of the coactivator CBP in p53 transcriptional activation. Mol Cell. 2004;13:251–263. doi: 10.1016/s1097-2765(03)00528-8. [DOI] [PubMed] [Google Scholar]
- 23.Wellings DA, Atherton E. Standard Fmoc protocols. Methods Enzymol. 1997;289:44–67. doi: 10.1016/s0076-6879(97)89043-x. [DOI] [PubMed] [Google Scholar]
- 24.Jeanmougin F, Wurtz JM, Le Douarin BP, Chambon P, Losson R. The bromodomain revisited. Trends Biochem Sci. 1997;22:151–153. doi: 10.1016/s0968-0004(97)01042-6. [DOI] [PubMed] [Google Scholar]
- 25.Manning ET, Ikehara T, Ito T, Kadonaga JT, Kraus WL. p300 forms a stable, template-committed complex with chromatin: role for the bromodomain. Mol Cell Biol. 2001;21:3876–3887. doi: 10.1128/MCB.21.12.3876-3887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–1333. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- 27.Rasband WS. Image J. U.S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2007. Available from: < http://rsb.info.nih.gov/ij/>. [Google Scholar]
- 28.Pearson-White SH. Human MyoD: cDNA and deduced amino acid sequence. Nucleic Acids Res. 1991;19:1148. doi: 10.1093/nar/19.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamonnak N, Fatkins DG, Wei L, Zheng W. N(epsilon)-methanesulfonyl-lysine as a non-hydrolyzable functional surrogate for N(epsilon)-acetyl-lysine. Org Biomol Chem. 2007;5:892–896. doi: 10.1039/b617185k. [DOI] [PubMed] [Google Scholar]