Abstract
The ability of embryos to diversify and of some adult tissues to regenerate throughout life is directly attributable to stem cells. These cells have the capacity to self-renew—that is, to divide and to create additional stem cells—and to differentiate along a specific lineage. The differentiation of pluripotent embryonic stem cells along specific cell lineages has been used to understand the molecular mechanisms involved in tissue development. The often endless capacity of embryonic stem cells to generate differentiated cell types positions the field of stem cells at the nexus between developmental biologists, who are fascinated by the properties of these cells, and clinicians, who are excited about the prospects of bringing stem cells from bench to bedside to treat degenerative disorders and injuries for which there are currently no cures. Here we highlight the importance of mice in stem cell biology and in bringing the world one step closer to seeing these cells brought to fruition in modern medicine.
Embryonic stem cells as a source for cell replacement therapy
Mouse embryonic stem cells (ESCs) were first isolated by in vitro culture of cells isolated from the inner cell mass (ICM) of early embryos or blastocysts1,2 (Fig. 1). Under the appropriate culture conditions, ESCs can proliferate indefinitely while retaining the ability to differentiate into all types of somatic cell (Fig. 2). When cultured ESCs are introduced into the ICM of mouse embryos, which are then transferred into the uterine duct of a foster mother mouse, the resulting offspring have chimeric tissues and organs composed of cells that derive partly from ESCs and partly from the ICM. Because ESC-derived germ cells are also present in the chimeric founder mice, this is a powerful approach for introducing specific genetic changes into the mouse germ line3.
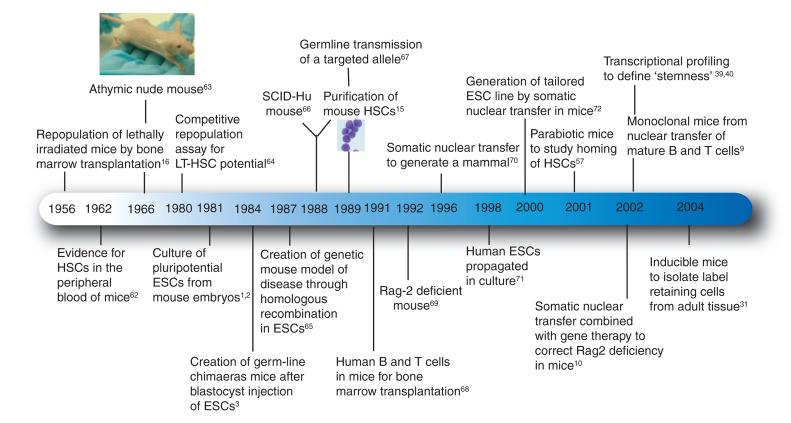
Figure 1.
Time line of principal discoveries in mouse stem cell research. Shown are many important discoveries made in the past 50 years as researchers have used mice as model systems for setting the foundations of stem cell biology. This work has been fundamentally important in bringing stem cell research to a clinical setting. LT-HSC, long-term HSC; SCID-Hu, severe combined immunodeficiency human.
Figure 2.
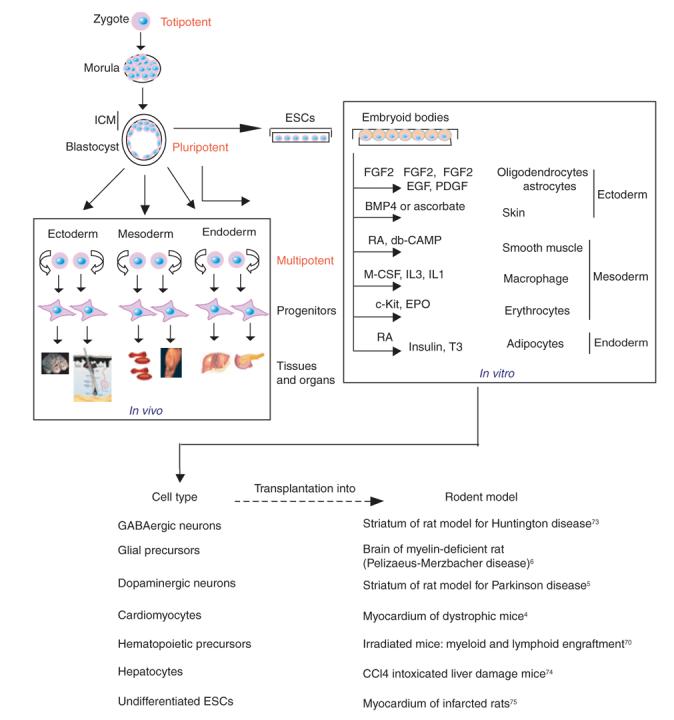
Coaxing ESCs down selective lineages for therapeutic application to injuries and degenerative disorders. Zygotes and their early cell divisions up to the morula stage are defined as totipotent because they can generate the whole mouse. At the blastocyst stage, only the cells of the ICM retain the capacity to generate all three primary germ layers (ectoderm, mesoderm and endoderm) that develop into the organs and tissues of the body. The ESCs cultured from the ICM of a blastocyst can be differentiated in vitro as embryoid bodies. Given the proper combination of growth factors, these embryoid bodies can differentiate into diverse types of cell. The resulting ESC-derived, differentiated mouse cells have been used in transplantation experiments in rodent models for injuries and degenerative disorders. BMP4, bone morphogenetic protein 4; Db-CAMP, dibutyryl cyclic AMP; EGF, epidermal growth factor; EPO, erythropoietin; FGF2, fibroblast growth factor 2; IL, interleukin; M-CSF, macrophage colony-stimulating factor; PDGF, platelet-derived growth factor; RA, retinoic acid; T3, triiodothyronine.
In the current era of ‘regenerative medicine’, scientists are now focused on optimizing the culture conditions necessary to coax cultured ESCs to differentiate into specific cell types such as cardiac, neural or endocrine lineages (Fig. 2). If a desired cell type can be produced en masse as a pure population in vitro, the cells can be tested for their potential to repopulate and to repair damaged or degenerating tissues. Emerging results from mouse models using lineage-specific cells generated from ESCs are promising and suggest that ESC-based cell replacement therapy might be applicable to treating human degenerative diseases associated with a loss or diminished pool of a particular cell type.
The potential for stem cell therapy extends to many disorders, including myocardial infarction4, Parkinson disease5, myelin disease6 and liver failure7 (Fig. 2). Many of the mouse studies involving regeneration therapy using ESC-derived cells are at a preliminary stage, and in most cases, the potential for long-term survival of the mice or for complicating side effects has not been analyzed in detail. But the successes achieved so far are inspiring and suggest that technologies pertaining to ESC-based regenerative therapies could be useful in a clinical setting.
Somatic cell nuclear transfer
One of the greatest challenges in harnessing the clinical potential of ESCs is to remove immunological barriers to the transplantation of tissues derived from human ESCs. The use of genomic replacement, known as somatic cell nuclear transfer, offers a possible solution to immune rejection of tissue-specific cell populations derived from the differentiation of foreign ESCs. Reprogrammed somatic cell therapy, called therapeutic cloning, uses this technology to introduce the nucleus of an adult donor cell (e.g., that of a B or T lymphocyte8) into an enucleated host ESC to generate a hybrid ESC line. Now tailored to be genetically matched to the donor, this ESC line can be differentiated into a selected cell type and used in regenerative transplantation to treat the affected individual. One concern is that the mitochondrial genome will still be derived from the initial ESC line, and so ‘foreign’ mitochondrial proteins will be made by these cells. Mouse studies will be useful to explore in greater detail the extent to which these foreign proteins could trigger an immune reaction. Such studies will be a prerequisite to applying this technology to human medicine.
These caveats aside, how well does the technology of nuclear transfer work? So far, most studies have centered on nuclear transfer from somatic cells to mouse eggs. This method produces hybrid ESCs called nuclear transfer ESCs (ntESCs), which are then introduced into the ICM of a host blastocyst to generate a ‘cloned’ mouse rather than being used for differentiation and regenerative therapy. Cloned mice have been generated by this approach, indicating that the necessary technical manipulations can be done without damaging the ability of the ntESCs to generate all the tissues and organs of the mouse9. What have the mouse models told us so far about the promises versus the pitfalls?
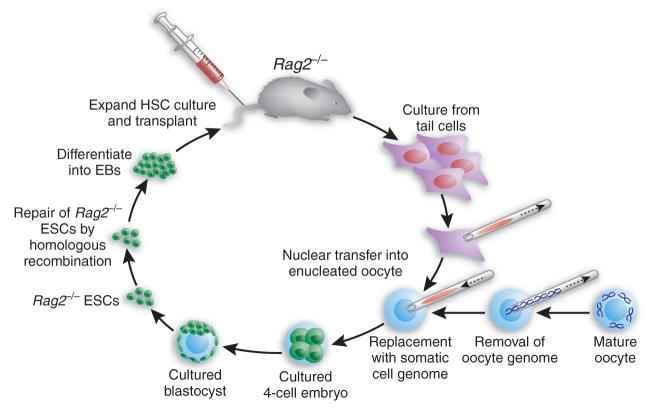
In a particularly creative example, Rideout et al.10 combined therapeutic cloning with gene therapy to correct a genetic defect in the immune system (Fig. 3). In this approach, nuclear transfer was first used to generate ntESCs that carried a mutation in the gene encoding the Rag2 recombinase, which is necessary for B- and T-cell development. Homologous recombination was then used to correct the defect in Rag2 in the cultured ntESCs. After their repair, the ntESCs were used to generate mice, which showed a complete restoration of immune function. Perhaps even more important for therapeutic cloning, the repaired ESCs were differentiated in culture to make hematopoietic stem cells (HSCs), which were then shown to rescue, at least partially, irradiated Rag2−/− mice10. This work represents a proof of principle for using somatic cell nuclear transfer therapy to treat a genetic disorder. Unexpectedly, however, the initial attempts at engraftment with these cells failed owing to an increase in natural killer cells. In this regard, more promising results have been obtained with the differentiation of ntESCs into a defined cell type that does not have the multiplicity of options imparted to stem cells, including HSCs. For example, the transplantation of ntESC-derived dopaminergic neurons seems to correct the phenotype of a mouse model of Parkinson disease11.
Figure 3.
Gene therapy combined with therapeutic cloning. Experimental scheme of Rideout et al.10, who used ESC technology and gene therapy to correct genetic defects in the hematopoietic system. Cell cultures are first made from tail tips of immunodeficient mice that are homozygous with respect to a knockout mutation in Rag2. Nuclei from these cells are then transferred into enucleated oocytes isolated from a normal mouse. The resulting hybrid cells are cultured and introduced into the ICM of mouse blastocysts to generate Rag2−/− ESCs or ntESCs. The Rag2 genetic mutation is then corrected by homologous recombination, and the manipulated ntESCs are differentiated in vitro first into embryoid bodies (EBs) and then into hematopoietic precursor cells, which are subsequently reintroduced into the original Rag2−/− mice to cure them.
In the future, it will be important to produce pluripotent ntESCs from the nuclei of individuals affected with different human genetic disorders and to use these pluripotent lines to study the development and pathogenesis of these diseases in vitro. Eleven human ESC lines were recently established by the nuclear transfer of skin cells obtained from individuals with disease or injury into donated oocytes12. In addition, recent studies suggest that human ESCs fused to somatic fibroblasts can reprogram the fibroblast nuclei and render the cells pluripotent13.
Adult stem cells from bench to bedside
Most, if not all, adult tissues set aside reservoirs of stem cells for replenishing cells that are lost in either tissue injury or homeostasis. Every time a mild wound is incurred, adult stem cells (ASCs) are called into action: hair follicle stem cells repair the damaged epidermis and hair follicles, and HSCs replenish the lost blood. ASCs are used sparingly and are generally tucked away in protected niches, where they exist as quiescent, undifferentiated and under-represented components of a tissue14. The isolation and characterization of ASCs pose challenges not only because of their minority status, but also because of the paucity of defining cell-surface markers.
The first multipotent ASCs to be purified and characterized were HSCs located in the bone marrow of mice15. These ASCs can reconstitute the hematolymphoid system of lethally irradiated mice16. The presence of HSCs in the bone marrow of both mice and humans has established the use of bone marrow transplants as a therapy to treat individuals with aplastic anemia, leukemias17, solid tumors, immune deficiency and hemoglobin disorders.
In subsequent years, other candidate ASCs have been defined only provisionally by their anatomical characteristics and slow-cycling properties. These candidates include muscle satellite cells, which are present beneath the basal lamina of mature muscle fibers18; skin epithelial stem cells, which are found below the sebaceous gland in the hair follicle in a region known as ‘the bulge’19; intestinal stem cells located near the base of the intestinal crypts20; cardiac stem cells21,22; neural crest stem cells23,24; and bronchioalveolar stem cells in the lung25.
ASCs provide the potential for correcting defective tissues and organs by autologous therapies, which eliminate the risk of graft rejection. Similar to the ESC-based strategies outlined above, there are numerous mouse models of disease showing that, after ex vivo genetic modification, autologous HSCs can restore a specific defect in a hematopoietic lineage. Such studies have been important in developing and improving methods to treat various human hematological disorders including cancers (Fig. 1).
Another therapeutic option for some diseases is the introduction and expression of recombinant genes in somatic cells (i.e., gene therapy) by using viral and nonviral vectors. Among the longest and most widely publicized trials are those directed towards children suffering from severe combined immunodeficiencies in which T-cell differentiation is arrested26. There are ten genetically distinct types of severe combined immunodeficiency, all of which lead to early death in the absence of therapy. The preliminary results of gene therapy using allogenic HSCs seemed very promising because many individuals responded well. Subsequently, however, 3 individuals from a trial of ∼13 treated developed T-cell leukemia. A chief risk associated with retrovirus-mediated gene transfer is the random insertion of retrovirus into proto-oncogenes of the genome. Indeed, the leukemic cells isolated from the affected individuals contained a viral insertion near an oncogene that is known to be activated by chromosomal translocation27.
Concomitant with the development of HSCs for treating immunodeficiency disorders came the development of epidermal stem cells for treating severely burned individuals28. In studies preceding human trials, immunocompromised mice were used as surrogates for cultured epidermal cell grafts, which produced and sustained a healthy patch of human epidermis. Although the skin generated from cultured epidermal grafts does not sweat or produce hair, it does save the lives of individuals with burns and is still in use in many countries.
After nearly two decades of further extensive studies on mice, researchers recently developed methods to isolate and to characterize multi-potent stem cells from the hair follicle29-31. One strategy has been to isolate stem cells on the basis of their slow-cycling characteristics and their expression of a fluorescently tagged transgene31. To generate a universal model for isolating infrequently cycling cells, a transgenic mouse has been engineered in which expression of histone H2B tagged with green fluorescent protein (GFP) is placed under the control of a tetracycline-responsive regulatory element (TRE). This mouse can be mated to any transgenic mouse expressing a TRE-binding transcriptional repressor (tetracycline-off) under the control of any cell type–specific promoter. To test the model, the keratin-5 promoter was used to target tetracycline-off to keratinocytes of the skin and other stratified epithelial tissues31. By administering doxycycline to the mice through their diet, H2B-GFP expression was shut off such that after 4–8 weeks only the infrequently cycling stem cells retained the label. Fluorescence-activated cell sorting was then used to isolate the stem cells.
For situations where little is known about ASCs of a tissue and, in particular, where diagnostic cell-surface markers have not been identified, the TRE-H2B-GFP mouse model might be useful if a suitable promoter to drive tetracycline-off is available. Identifying, purifying and monitoring the slow-cycling, label-retaining cells would enable an investigation of the extent to which the cells might be stem cells. In engraftment experiments, keratinocytes cultured from a single follicle stem cell can generate a patch of fluorescent epidermis, sebaceous glands and hairs on an otherwise nude athymic mouse29. Although it is far too early to judge whether the technology for restoring hair on the back of a nude mouse will translate into a clinical treatment for human hair disorders, the ability to determine the transcriptional profile of these cells paves the way for elucidating the mechanisms by which the epithelial stem cells of the skin can respond to external cues to induce a new round of hair growth.
The successes in translating ASCs to the clinic have intensified the search for ASCs in other tissues. This interest has been sparked, in part, by the ethical controversies surrounding the potential use of ESCs in therapies for human diseases. When it comes to treating a disorder of a defined cell type, however, the corresponding ASC can be useful because of its more restricted potential, providing that it can be cultured in vitro and coaxed to follow a particular lineage. Although very few ASCs have been successfully propagated in vitro, the availability of the transcriptional profiles of many ASCs has identified their repertoire of cell-surface receptors, which may help to improve culture conditions in the future.
What other ASCs are on the horizon? Multipotent cells in nerve tissue from adult mouse brain have been purified and can divide and give rise to both neurons and glial cells in culture24,32. In addition, transplantation of enriched neural populations has shown clinical utility in treating monocellular neurological disorders such as Parkinson disease, which is caused by the degradation of dopaminergic neurons in the striatum33. The survival rate of grafted dopaminergic neurons derived from the expanded precursor is low (3–5%), however, which suggests that improved cell survival is needed after grafting.
The pancreas shows limited cell turnover, and an endogenous source of stem cells has been proposed to regenerate pancreatic insulin-secreting cells34. The pancreas contains insulin-secreting islets that modulate glucose levels in humans and are destroyed by autoimmune attack in type 1 diabetes. Because the replenishment of pancreatic β-cell populations is accompanied by endogenous insulin secretion, the isolation of pancreatic stem cells that can produce new β-cells could lead to a cure for type 1 diabetes. Recent work shows in mice, however, that pre-existing β-cells rather than multipotent stem cells are the principal source of new β-cells in adult life and after pancreatectomy35. This work suggests that differentiated cells of a tissue may retain proliferative capacity in vivo.
Skeletal muscle provides an interesting example for biologists who traditionally seek out stem cells in a particular niche or reservoir. The mitotically quiescent myogenic precursors are called satellite stem cells. Although satellite stem cells have received considerable attention for their scope in treating individuals affected with Duchenne muscular dystrophy, the functional properties of these muscle-derived stem cells remain unclear. A clonal population of muscle progenitor cells has been identified and, after intramuscular or intravenous injection, results in muscle regeneration and partial restoration of dystrophin in mdx mice, a model of Duchenne muscular dystrophy36. After intra-arterial injection, isolated muscle progenitors seem to engraft dystrophic mouse muscle and to participate in regeneration after muscle damage37,38. Whether any of these cultured cells represent a pure stem cell pool remains unclear; nevertheless, myogenic cell lines may be useful as a source of myogenic progenitors for direct or systemic transplantation.
Analyzing the properties of putative stem cell genes
With the advent of gene array technology, the molecular characterization of stem cells has taken a leap forward, providing comprehensive snapshots of the transcriptional profiles of these cells. This progress has prompted researchers to address some fundamental questions about stem cell biology. Are certain features common to all stem cells? How do ASCs differ from ESCs? What are the molecular mechanisms that control the quiescence state of stem cells and their ability to undergo self-renewal? And how are stem cells activated in their conversion from a quiescent, multipotent cell to a committed cell that transiently divides and differentiates along a particular lineage?
Two main challenges confront researchers trying to harness the transcriptional repertoire of multipotent stem cells. The first is devising strategies for isolating and purifying these minority components of a tissue; the second is choosing what to compare these cells with. In early studies of this kind, Ivanova et al.39 used cell-surface markers and fluorescence-activated cell sorting to isolate and to determine the transcriptional profile of HSCs and their early committed transient amplifying progeny. Ramalho-Santos et al.40 used similar methods to isolate HSCs but compared their transcriptional profiles to that of whole bone marrow. Despite reproducible array data for the HSCs, the list of what constitutes HSC ‘stemness’ differed markedly in the two studies. One group defined genes that were upregulated in HSCs relative to the whole heterogeneous tissue surrounding the cells, whereas the other defined genes whose expression altered as HSCs changed their properties and embarked on a lineage. Both types of comparison are useful, but they yield very different information.
As an ever-increasing number of stem cells and their progeny are being isolated and profiled, detailed expression data are accumulating at a rapid rate. Scientists have begun to reap the benefits of these molecular signatures by probing more deeply into the mechanisms by which stem cells are maintained in their niches in quiescent and differentiation-inhibited states and how they become mobilized as they exit their niches and select a particular lineage.
Many examples show how molecular markers, sometimes coupled with transgenic and knockout mouse models, have offered glimpses into the mechanisms underlying the maintenance and self-renewal of stem cells. Hoxb4, for example, is a homeodomain-containing transcription factor that has surfaced in several different arrays. Hoxb4 extends viability and proliferation when overexpressed in mouse HSCs41, which in turn yield improved efficiency of rescue when transplanted into HSC-depleted mice. Another example is Bmi-1, a member of the chromatin-regulating Polycomb complex, which is required for generating self-renewing adult HSCs42 and for the self-renewal of neural stem cells43.
Equally as important as chromatin remodeling factors in stem cell governance are the signal transduction pathways involved. Wnt signaling has surfaced as a universal regulator of stem cells, but exactly how Wnts act on stem cells remains a matter of intense debate44. Cells respond to canonical Wnt signaling by stabilizing excess β-catenin that is not used in cell-cell adhesion. This stabilized β-catenin is then free to associate with, and to act as a transcriptional cofactor for, members of the Lef1 or Tcf family of DNA-binding proteins. Transgenic overexpression of a constitutively stabilized version of β-catenin in skin epidermis yields de novo hair follicle morphogenesis, a feature that is characteristic of multipotent embryonic skin stem cells but not of adult epidermis45. Slightly lower levels of Wnt signaling leads to ASC activation of existing hair follicles46. Conversely, loss-of-function mutations in β-catenin completely block hair follicle formation47. In the intestine, loss of the β-catenin partner Tcf4 blocks tissue formation48.
In the nervous system transgenic overexpression of β-catenin results in enlarged brains and expansion of the precursor population49, whereas in neural crest stem cells stabilized β-catenin has little effect on the population size and instead regulates fate decisions50. In bone marrow progenitors, inactivation of β-catenin in vivo does not seem to impair the ability of these cells to self-renew and to reconstitute hematopoietic lineages51. But overexpression of β-catenin in cultured descendents from isolated HSCs promotes the proliferation of these cells, which, when injected into the blood of lethally irradiated mice, at least sustain the production of myeloid, B- and T-cell lineages52.
While researchers are sifting through the mechanisms underlying Wnt signaling in stem cell biology, so too are they exploring how other pathways may act in concert to integrate stem cell biology. Similar to Wnt signaling, Notch and bone morphogenetic protein signaling seem to have pleiotropic effects on stem cells and their lineages, and these effects seem to differ for distinct stem cells53. Whether these factors operate at the level of stem cell self-renewal, stem cell activation, or transient amplifying cell proliferation or differentiation remains unclear. For example, tissue impairment observed in mouse knockout models could arise either from a defect in stem cells or from the failure of transient amplifying cell progeny to proliferate. Similarly, the effects of a signaling factor on the proliferation of cultured stem cells could be either a sign of enhanced proliferation of transient amplifying cell progeny within the culture or an indication of self-renewal of stem cells. As future studies are carried out, it should become apparent whether stem cells are maintained and propagated by a common set of regulatory pathways and molecular principles, or whether the seemingly opposing effects of signaling molecules on the behavior of different cells is a reflection of the diversity of ASCs.
Stem cells at home
In adult tissues, multipotent stem cells are often tucked away in protected places or niches. The most protected niche is the bone marrow, which is the location of HSCs. The skin can be microdissected into units in which every hair follicle has a tiny niche (the bulge) of stem cells, which are responsible for generating a new hair during the hair cycle and for replenishing the cells of the sebaceous gland and the epidermis on injury54. Similarly, the intestine is composed of hundreds of units each containing a villus and a crypt, and the stem cells are located in a ring just above the base of the crypt. The stem cells in each crypt are responsible for generating the four lineages of absorptive and secretory cells that constitute the crypt and villus20. In the adult central nervous system, the stem cells are located in the subventricular zone, where they can generate glia and neurons24.
The importance of the niche is best exemplified by experiments in which the fate of ESCs is monitored after their subcutaneous injection into immunodeficient nude mice. When placed into a foreign environment of surrounding in vivo tissue, ESCs form benign teratomas that contain a multiplicity of cell types55. This study shows the importance of an appropriate microenvironment of specific intercellular interactions and cellular organization.
Most stem cells commit to differentiate along specific lineages when they leave their niche. HSCs seem to be an exception because they can circulate through the bloodstream and find their way back home relatively unscathed in a process called homing56. Experiments using genetically marked CD45.1 and CD45.2 parabiotic mice, which share a common circulation, have been done to study the migration of HSCs from the bone marrow though the blood57. The results show that HSCs detectable in the peripheral blood have migrated out of their niche, leading to the possibility that blood-borne HSCs may be a source of pluripotent or multipotent stem cells available for the repair of both nonhematopoietic and hematopoietic tissues57.
Because stem cells depend on their surrounding environment to maintain their stem cell properties, the development of the niche is crucial14. Inside the niche, stem cells typically maintain a growth- and differentiation-inhibited environment. Every time a stem cell is prompted to exit the niche, it must be replenished, presumably by self-renewal. Conversely, for a stem cell to leave the niche and follow a particular lineage, its microenvironment must change or accumulate or lose essential factors that otherwise keep it in its niche.
Genetic studies on germ cells in Drosophila melanogaster provide the best picture of how this change might happen58. Although research into mammalian niches lags behind, the recent characterization and genetic manipulation of mouse ASCs and their niches have led to advances in our understanding of what constitutes this environment in mammalian stem cell biology. Such studies have also given insights into how key signaling pathways orchestrate stem cell behavior.
Among the more intriguing advances in this field are explorations into the depths of the HSC bone marrow niche that is lined with spatially oriented osteoblasts. As HSCs progressively mature, they lose contact with these neighboring stromal cells, become more proliferative, head toward the central bone marrow cavity and traverse into the blood vessels. When mice are genetically altered to increase the osteoblast population in the inner surface of bone, HSC numbers rise concomitantly and the ability of these new HSCs to retain their slow-cycling properties seems to rely on their capacity to adhere directly to osteoblasts through N-cadherin–mediated adherens junctions59. These studies have identified a crucial role for osteoblasts in maintaining the long-lived, most valuable HSCs. Evidence also shows that at least some HSCs contact blood vessel sinusoidal endothelium in the bone marrow, which may explain why HSCs can be mobilized into the circulation with very rapid kinetics60. Notably, embryonic skin stem cells have recently been shown to use adherens junctions in controlling asymmetric cell division, which may have broad implications for stem cells61.
In conclusion, the characterization and manipulation of stem cells in mice have led to a wide range of clinical applications and promising avenues for future research. In bringing this powerful technology from the realm of mouse models to the clinical setting, scientists worldwide will need to develop guidelines that balance moral and ethical concerns with benefits to therapeutic medicine. In so doing, the onus is on scientists to communicate effectively and to convince the public of the importance of moving forward.
ACKNOWLEDGMENTS
We thank M. Schober, V. Horsley and C. Blanpain for discussions and advice during the preparation of this review. G.G. is a recipient of Human Frontier Science Program. E.F. is an Investigator of the Howard Hughes Medical Institute and the recipient of funding from the US National Institutes of Health.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 4.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embryonic stem cells form stable intracardiac grafts. J. Clin. Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JH, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 6.Brustle O, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 7.Chinzei R, et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36:22–29. doi: 10.1053/jhep.2002.34136. [DOI] [PubMed] [Google Scholar]
- 8.Hochedlinger K, Jaenisch R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature. 2002;415:1035–1038. doi: 10.1038/nature718. [DOI] [PubMed] [Google Scholar]
- 9.Wakayama T, et al. Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science. 2001;292:740–743. doi: 10.1126/science.1059399. [DOI] [PubMed] [Google Scholar]
- 10.Rideout WM, III, Hochedlinger K, Kyba M, Daley GQ, Jaenisch R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002;109:17–27. doi: 10.1016/s0092-8674(02)00681-5. [DOI] [PubMed] [Google Scholar]
- 11.Barberi T, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat. Biotechnol. 2003;21:1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- 12.Hwang WS, et al. Patient-specific embryonic stem cells derived from human SCNT blastocysts. Science. 2005;308:1777–1783. doi: 10.1126/science.1112286. [DOI] [PubMed] [Google Scholar]
- 13.Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 15.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 16.Ford CE, Hamerton JL, Barnes DW, Loutit JF. Cytological identification of radiation-chimaeras. Nature. 1956;177:452–454. doi: 10.1038/177452a0. [DOI] [PubMed] [Google Scholar]
- 17.Buckner CD, et al. Allogeneic marrow engraftment following whole body irradiation in a patient with leukemia. Blood. 1970;35:741–750. [PubMed] [Google Scholar]
- 18.Seale P, Asakura A, Rudnicki MA. The potential of muscle stem cells. Dev. Cell. 2001;1:333–342. doi: 10.1016/s1534-5807(01)00049-1. [DOI] [PubMed] [Google Scholar]
- 19.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 20.Loeffler M, Birke A, Winton D, Potten C. Somatic mutation, monoclonality and stochastic models of stem cell organization in the intestinal crypt. J. Theor. Biol. 1993;160:471–491. doi: 10.1006/jtbi.1993.1031. [DOI] [PubMed] [Google Scholar]
- 21.Beltrami AP, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 22.Laugwitz KL, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchstaller J, et al. Efficient isolation and gene expression profiling of small numbers of neural crest stem cells and developing Schwann cells. J. Neurosci. 2004;24:2357–2365. doi: 10.1523/JNEUROSCI.4083-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Cavazzana-Calvo M, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 27.Hacein-Bey-Abina S, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 28.Gallico GG, III, O'Connor NE, Compton CC, Kehinde O, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N. Engl. J. Med. 1984;311:448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 29.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 31.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rietze RL, et al. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 33.Studer L, Tabar V, McKay RD. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat. Neurosci. 1998;1:290–295. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- 34.Seaberg RM, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 35.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 36.Lee JY, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J. Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torrente Y, et al. Intraarterial injection of muscle-derived CD34+Sca-1+ stem cells restores dystrophin in mdx mice. J. Cell Biol. 2001;152:335–348. doi: 10.1083/jcb.152.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins CA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Ivanova NB, et al. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 40.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. ‘Stemness’: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 41.Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- 42.Park IK, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 43.Molofsky AV, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr. Opin. Cell Biol. 2004;16:681–687. doi: 10.1016/j.ceb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 46.Lowry WE, et al. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 48.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 49.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 50.Lee HY, et al. Instructive role of Wnt/β-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- 51.Cobas M, et al. β-Catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reya T, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 53.Duncan AW, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 54.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 55.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 56.Quesenberry PJ, Colvin G, Abedi M. Perspective: fundamental and clinical concepts on stem cell homing and engraftment: a journey to niches and beyond. Exp. Hematol. 2005;33:9–19. doi: 10.1016/j.exphem.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 58.Yamashita YM, Fuller MT, Jones DL. Signaling in stem cell niches: lessons from the Drosophila germline. J. Cell Sci. 2005;118:665–672. doi: 10.1242/jcs.01680. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 60.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 61.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodman JW, Hodgson GS. Evidence for stem cells in the peripheral blood of mice. Blood. 1962;19:702–714. [PubMed] [Google Scholar]
- 63.Flanagan SP. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 1966;8:295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- 64.Harrison DE. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 65.Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 66.McCune JM, et al. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 67.Thompson S, Clarke AR, Pow AM, Hooper ML, Melton DW. Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell. 1989;56:313–321. doi: 10.1016/0092-8674(89)90905-7. [DOI] [PubMed] [Google Scholar]
- 68.Lubin I, et al. Engraftment and development of human T and B cells in mice after bone marrow transplantation. Science. 1991;252:427–431. doi: 10.1126/science.1826797. [DOI] [PubMed] [Google Scholar]
- 69.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 70.Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- 71.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 72.Munsie MJ, et al. Isolation of pluripotent embryonic stem cells from reprogrammed adult mouse somatic cell nuclei. Curr. Biol. 2000;10:989–992. doi: 10.1016/s0960-9822(00)00648-5. [DOI] [PubMed] [Google Scholar]
- 73.Dinsmore J, et al. Embryonic stem cells differentiated in vitro as a novel source of cells for transplantation. Cell Transplant. 1996;5:131–143. doi: 10.1177/096368979600500205. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto H, et al. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology. 2003;37:983–993. doi: 10.1053/jhep.2003.50202. [DOI] [PubMed] [Google Scholar]
- 75.Min JY, et al. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J. Appl. Physiol. 2002;92:288–296. doi: 10.1152/jappl.2002.92.1.288. [DOI] [PubMed] [Google Scholar]