Summary
While newer neuraminidase inhibitors have been used recently to treat influenza A and B virus infections, emergence of drug resistance poses potential problems. Previous ribavirin aerosol treatments of influenza were effective and drug resistance was not observed. To make ribavirin aerosol treatment a quicker process and limited to once or twice daily treatments, a MegaRibavirin formulation (100 mg of ribavirin/mL) was developed that when used with the Aerotech II nebulizer was effective in preventing death in a lethal influenza A virus mouse model. Aerosol generated using the Aerotech II nebulizer flowing at 10 L of air/min produced aerosol droplets that contained 2.3 mg of ribavirin/L with a mass median aerodynamic diameter of 1.8 μm. Using this system for treatment, a single daily 30-min exposure on days 1 to 4 produced a survival rate of greater than 90%. Delaying the start of aerosol treatment for 48 or 72 h and treating once daily for 30 min for two days (days 2−3 and 3−4, respectively) still significantly increased the number of survivors and mean time to death. For the treatment of influenza in general and for pandemic avian influenza, the MegaRibavirin-Aerotech II method of aerosol treatment allows for short treatment periods, minimizes environmental issues and costs less.
Keywords: Aerosol, Ribavirin, Influenza, Mice, Treatment
1. Introduction
In the early 1980's, clinical trials of ribavirin aerosol for the treatment of naturally occurring influenza in college students were conducted (Gilbert et al., 1985;Gilbert and Knight, 1990;Knight et al., 1981;McClung et al., 1983;Wilson et al., 1984). The results of those studies showed that ribavirin administered as an aerosol was effective in reducing nasal wash virus titers, duration of fever (>100°F) and symptomatology (Gilbert and Knight, 1990;Knight and Gilbert, 1987). Dosing and treatment schedules were based on studies of ribavirin aerosol treatment of influenza-infected mice (Gilbert et al., 1991, 1992;Wyde et al., 1986, 1987). Initially, treatment of influenza utilized 20 mg of ribavirin/mL in the reservoir of a prototype of the SPAG2−6000 (SPAG) nebulizer from ICN Pharmaceuticals and continuous exposure to the aerosol for 12 h. With FDA approval of ribavirin aerosol for the treatment of respiratory syncytial virus (RSV) infections in hospitalized children, the long, continuous treatment requirements interfered with patient care. Using animal models, it was shown that by increasing the ribavirin concentration in the reservoir three-fold, the time of treatment could be reduced significantly. Thus, with 60 mg of ribavirin/mL in the reservoir (“high dose”) of a SPAG nebulizer, the time of ribavirin aerosol treatment of influenza A or B virus infection could be reduced to three 15-min exposures, and for RSV to three 30-min exposures (Gilbert et al., 1992;Wyde et al., 1987). Clinically, this approach has also been used in children with suspected RSV infections (Englund et al., 1990, 1994) and in bone marrow transplant patients infected with RSV. These patients were treated with “high dose” ribavirin for 2 h, three-times daily (Boeckh et al., 2007;Whimbey et al., 1995). To date results with ribavirin aerosol treatment of these viral infections has translated into clinical effectiveness.
While newer anti-influenza drugs such as the neuraminidase inhibitors are available, drug resistance has developed in the clinical setting to these drugs. However, even with use of ribavirin for the treatment of RSV by aerosol, hepatitis C virus infections by oral administration and hemorrhagic fever viruses by intravenous administration, resistance to ribavirin has not been reported. We believe that this newer methodology for treating influenza is applicable to influenza in general but would be particularly valuable in treating pandemic avian influenza where shorter treatment times and the need for one-third or one-sixth of the drug would be advantageous. Most likely a combination of antivirals will be necessary and with different modes of action, a neuraminidase inhibitor and ribavirin aerosol should be likely candidates.
In the last two decades, advances in formulation and nebulizers have occurred that produce higher ribavirin aerosol concentrations leading to higher deposited doses when delivered for even shorter periods of time than the “high dose” formulation. In this manuscript, we describe the delivery of “MegaRibavirin”, a 100 mg of ribavirin/mL formulation using a more efficient nebulizer, the Aerotech II. A 30-min, once daily aerosol treatment starting 24 hours post-infection and continuing for 3 or 4 days effectively protected mice from a lethal influenza A/HK/8/68 (H3N2) virus infection.
2. Materials and methods
2.1 Animals
NIH Swiss-Webster female mice, 6−8 weeks of age (18−20 g), were obtained from NCI-Charles Rivers Laboratories (Hartford, CT). They were housed in the Baylor College of Medicine (BCM) vivarium in cages covered with barrier filters and given food and water ad libitum. Experiments were performed utilizing NIH and United States Department of Agriculture guidelines and experimental protocols approved by the BCM Investigational Animal Care and Use Committee (IACUC).
2.2 Virus inoculation and quantification
Mice were inoculated with mouse-adapted influenza A/HK/8/68 (H3N2) virus by small-particle aerosol as described previously (Gilbert et al., 1992;Wyde et al., 1977). Originally, the virus was obtained from a patient experiencing influenza. It was passaged two times in rhesus monkey kidney tissue culture and then passaged serially in BALB/c mice as described previously (Wyde et al., 1977). The Aerotech II nebulizer (CIS-USA; Bedford, MA) is commercially available and has been used in clinical trials (Iacono et al., 2006; Verschraegen et al., 2004). This nebulizer can hold up to 10 mL of liquid and run at a flow rate of up to 10 L of gas/min. Briefly, 9 mL of a 1:300 dilution of a mouse lung pool of influenza A/HK/68 (H3N2) (stock titer of 7.43 TCID50/mL) in 0.05% gelatin-minimal essential medium (MEM) was added to the reservoir of an Aerotech II nebulizer flowing at 10 L of air/min produced by an Aridyne 2000 compressor (Trimeter Instrument Corp., Landcaster, PA). Mice were exposed to the aerosol for 20 min, depositing approximately 100 TCID50/mouse.
On day 4 post-influenza A/HK/8/68 (H3N2) virus inoculation (p.i.), entire lungs from five animals were each homogenized in 1 mL of MEM, diluted 1:10 with MEM and serially diluted 1:3 using MEM in 96-well round bottom plates of Madin Darby canine kidney (MDCK) cells containing MEM with Worthington trypsin (2 μg/mL) and without fetal bovine serum (FBS) as described previously (Wyde et al., 1987). After incubation for 5 days at 36°C and 5% CO2, 0.05 mL of a 0.5% suspension of chicken erythrocytes was added to each well. Wells exhibiting hemadsorption were considered to be infected with influenza virus. Virus titers were calculated as TCID50/lung.
2.3 MegaRibavirin aerosol characteristics
Ribavirin was resuspended in water at 100 mg/mL and 10 mL added to an Aerotech II nebulizer flowing at 10 L of air/min. To determine total aerosol concentrations (μg ribavirin/L of aerosol), samples (1 min) were collected in an All-glass impinger (AGI) (Ace Glass; Vineland, N.J.) containing 20 mL of water at 1, 7, 21 and 29 min of a 30-min exposure period. In addition to determine aerosol particle size, a sample (1 min) was collected at 15 min using an Andersen cascade impactor (Andersen Instruments; Atlanta, GA). Ribavirin from each plate of the impactor was eluted with 5 mL of water and the concentration was determined spectrophotometricly at 207nm. A standard curve (0−50 μg of ribavirin/mL) was used with an R2 of 0.9945 and a slope of 0.0430 A207nm/μg/mL. The mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) were calculated from these data (Wiggins, 1991). From the mean aerosol concentration (aerosol MT), the deposited dose of ribavirin in the lungs of mice (20.4 mg/kg/30 min exposure) was estimated as described previously (Knight et al., 1988;Knight and Gilbert, 1987;Koshkina et al., 2004).
2.4 MegaRibavirin aerosol treatment
Mice contained in sealed plastic cages were exposed to aerosolized drug for 30 min using the Aerotech II nebulizer flowing at 10 L of air/min as described previously (Gilbert and Wyde, 1988;Knight et al., 1999). Ribavirin (1 g powder) was mixed with 10 mL of sterile water for irrigation (Baxter Healthcare Corp.; Deerfield, IL), vortexed and then sonicated in a water bath for 15−30 s to assure complete solubilization. The final concentration of ribavirin was 100 mg/mL. A new nebulizer was used for each treatment. For quality control of performance, nebulizers were weighed before and after treatment to monitor the rate of liquid consumption during the treatment period. In general, mice were exposed to virus on day 0, treated with various dosing regimens usually starting 24 h post-infection (p.i.) (i.e., day +1), tested for virus on day +4 p.i. (day of peak virus titer) and observed for mean change in body weights and mortality until day +21.
2.5 Statistical analysis
The Fisher Exact test, two-tailed using Instat3® software was used to compare the final fraction of deaths in a group to that of another group. The Student's t-Test, two-tailed using Microsoft's Excel® software was used to compare between groups the mean time to death (MTD) of those that died and changes in mean body weights. ANOVA with Bonferroni multiple comparisons tests, two-tailed using Instat3® software was used to compare survival curves and pulmonary virus titers using log10/lung transformed data.
3. Results
3.1 MegaRibavirin aerosol characteristics
Ribavirin at a concentration of 100 mg/mL (MegaRibavirin) produced an aerosol containing droplets with mass median aerodynamic diameter (MMAD) of 1.76 μm and a geometric standard deviation (GSD) of 2.60 (Fig. 1A). Most (83.6%) of the droplets were in the respirable range (< 5 μm) and suitable for deep deposition into the lower airways. This droplet size was similar to previous measurements of the standard ribavirin concentration (20 mg/mL) used in the SPAG nebulizer for the treatment of influenza in college students (Gilbert et al., 1985;Knight et al., 1981;McClung et al., 1983;Wilson et al., 1984) and the FDA-approved treatment of RSV in hospitalized children (Taber et al., 1983). The mean aerosol concentration (aerosolMT) of ribavirin produced over a 30 min treatment period by the Aerotech II nebulizer was 2,277 μg/L of aerosol (Fig. 1B). The efficiency of the Aerotech II nebulizer with MegaRibavirin was 2.3 times greater in generating an aerosol concentration from the liquid reservoir than the SPAG nebulizer at 20 mg ribavirin/mL (Table 1). Using the Aerotech II nebulizer with MegaRibavirin, a 30 min treatment twice daily would produce the same deposited dose of ribavirin in the lungs mice or man as the single 12 h treatment with the SPAG and would only require 2 g of drug instead of 6 g.
Fig. 1.
Aerosol characteristics of MegaRibavirin (100 mg/mL) generated from an Aerotech II nebulizer flowing at 10 L of air/min. A. Ribavirin distribution by particle size as determined by collection on an Andersen cascade impactor, elution of drug from the plates for each particle size range and quantitation by spectrophotometer (absorption at 207 nm). B. Ribavirin concentrations in the aerosol and nebulizer reservoir during a 30-min nebulization with an Aerotech II nebulizer. MMAD, mass median aerodynamic diameter; GSD, geometric standard deviation; AerosolMT, mean aerosol concentration of ribavirin over the 30 min period (μg/L of aerosol).
Table 1.
Comparison of Ribavirin Pulmonary Deposition Calculations Between Humans and Mice Using Different Nebulizers and Concentrations
| Speciesa | Nebulizerb | Reservoir Ribavirinc | Aerosol Ribavirind (μg/L) | Treatment Schedulee (no. × h) | Body Weight (kg) | Minute Volumef (L-min/kg) | Deposition Fractiong | Ribavirin Deposited/Treatmenth | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/mL) | (mL) | (mg/kg) | (total mg) | |||||||
| Human | SPAG | 20 | 300 | 200 | 1 × 12 | 70 | 0.108 | 0.70 | 10.9 | 762 |
| Human | SPAG | 60 | 100 | 600 | 2 × 2 | 70 | 0.108 | 0.70 | 10.9 | 762 |
| Human | AT2 | 100 | 10 | 2,270 | 1 × 0.5 | 70 | 0.108 | 0.70 | 5.1 | 360 |
| Human | AT2 | 100 | 10 | 2,270 | 2 × 0.5 | 70 | 0.108 | 0.70 | 10.3 | 721 |
| Mouse | SPAG | 20 | 300 | 200 | 1 × 12 | 0.02 | 1.0 | 0.30 | 43.2 | 0.86 |
| Mouse | SPAG | 60 | 100 | 600 | 3 × 0.25 | 0.02 | 1.0 | 0.30 | 8.1 | 0.16 |
| Mouse | AT2 | 100 | 10 | 2,270 | 1 × 0.5 | 0.02 | 1.0 | 0.30 | 20.4 | 0.41 |
| Mouse | AT2 | 100 | 10 | 2,270 | 2 × 0.5 | 0.02 | 1.0 | 0.30 | 40.9 | 0.82 |
Calculations based on adult species using nose breathing. Values may vary with younger aged individuals or animals.
SPAG: SPAG2−6000 nebulizer currently used for ribavirin aerosol treatments and manufactured by Valeant Pharmaceuticals International, Aliso Viejo, CA; AT2: Aerotech II nebulizer currently used in clinical trials for cancer and lung transplantation and manufactured by CIS-USA, Bedford, MA.
Concentrations: 20 mg/mL, used with standard FDA-approved aerosol treatment; 60 mg/mL, used with “high dose” treatment (Englund et al., 1990); 100 mg/mL, MegaRibavirin treatment (this report).
From measured aerosol concentrations: The AT2 nebulizer produces an aerosol concentration 2.3-times greater than the SPAG nebulizer.
Multiple treatments are evenly spaced over a 24 hr period. Treatments are expressed as number (no.) of treatments/day times the hours (h) of treatment.
From Phalen (1984).
From Schlesinger (1985) and Knight et al. (1988).
Deposited Dose (mg/kg/treatment) = [(Aerosol, μg/L) × (Minute volume, L-min/kg) × (Treatment time, min) × (Fractional deposition)]/1000 (Knight and Gilbert, 1987)
3.2 Comparison of once or twice daily 30-min MegaRibavirin aerosol treatments of influenza A/HK/8/68 (H3N2) virus-infected mice
In two separate experiments, mice infected by aerosol (ca. 100 TCID50/mouse) were treated for 30 min with an aerosol containing ribavirin either once daily or twice daily every 12 h (Fig. 2). Treatments were administered on 1) day +1, 2) on days +2 and +3) and 3) on days +1, +2, +3 and +4. With either once or twice daily treatments, the most dramatic effect was observed when the animals were treated for 4 days starting 24 h p.i. (days +1 through +4). This was observed for both survival (P < 0.05, once daily; P <0.001, twice daily) (Fig. 2A) and mean virus lung titers with an average decrease of 1.3 log10/lung (P < 0.05) (Fig. 2B). Minimizing treatment to a single 30-min exposure on day +1-only, was not effective in this (Fig. 2) or other experiments (data not shown). However, with once daily treatment starting 48 h p.i. and treating for 2 days (days +2 and +3), there was a statistically significant increase in overall survival (P <0.05) (Fig. 2A) and in the mean time to death from 8.5+1.3 to 9.6+0.5 days (P = 0.041) although the fraction of survivors (P = 0.109) and decrease in mean lung virus titers (0.76 log10/lung) were not significantly different (P >0.05) from untreated control animals (Fig. 2B).
Fig. 2.
Comparison of once daily or twice daily every 12 h 30-min MegaRibavirin (100 mg/mL; Rib) aerosol treatments on survival and lung virus titers of influenza A/HK/8/68 (H3N2) virus-infected mice. A. Effect on survival. *, P value ≤ 0.05 for comparing the survival curves in each group to the untreated group in each of the experiments using ANOVA with Bonferroni multiple comparisons tests, two-tailed. B. Effect on lung virus titers. *, P value < 0.05 for comparing the lung virus titers (mean ± standard deviation) in each group to the untreated group using the Student's t-Test, two-tailed on log10/lung transformed data.
3.3 Comparison of the treatment schedule of once daily 30-min MegaRibavirin aerosol treatments on influenza A/HK/8/68 (H3N2) virus-infected mice
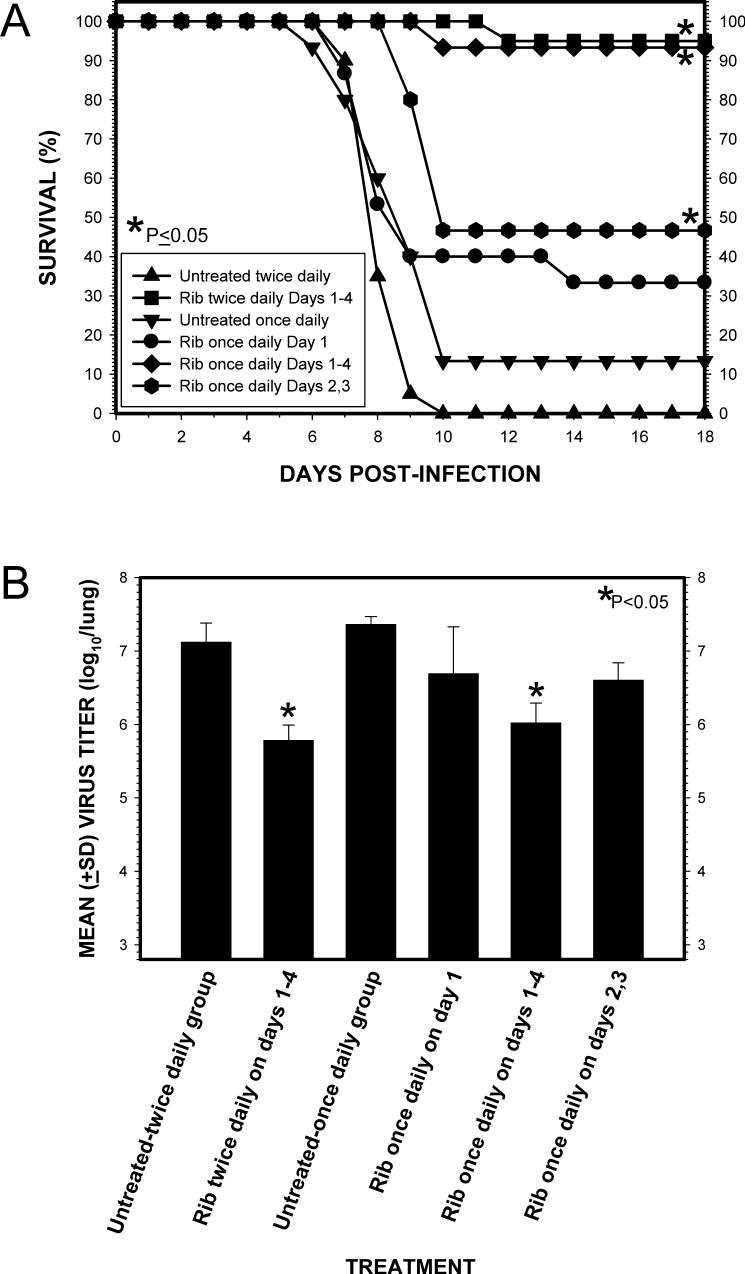
Mice were given a 30-min aerosol treatment once daily using different schedules to try to assess the minimum amount of treatment necessary to achieve an effective response (i.e., >90% survival rate) (Fig. 3). MegaRibavirin aerosol administered on 1) 3 days starting 24 h p.i. and treating on days +1, +2 and +3 or 2) for 2 days starting 72 hr p.i and treating on days +3 and +4 increased overall survival (P <0.05) (Fig. 3A), the fraction of survivors (P < 0.0001) and the mean time to death from 7.7±0.6 days to 9.4±1.1 and 9.8±1.2 days, respectively (P <0.001). Compared to the other groups, a decrease in mean body weight loss on days 7 and 9 was observed in the animals treated for 3 days (P <0.05) (Fig. 3B). In contrast, MegaRibavirin aerosol administered 1) for 2 days starting 24 h p.i. and treating on days +1 and +2 or 2) 48 h p.i. on days +2 and +3 had less of an effect on overall survival (P >0.05). Although there was a statistically significant increase in mean time to death in both, 9.5±1.2 and 9.0±1.7 days, respectively, compared to untreated controls, 7.7±0.6 days (P <0.004), the fraction of survivors was only significant for the mice treated on days +2 and +3 (P = 0.008).
Fig. 3.
Comparison of the treatment schedule of once daily MegaRibavirin (100 mg/mL) aerosol treatments on survival and changes in mean body weights of influenza A/HK/8/68 (H3N2) virus-infected mice. A. Effect on survival. *, P value ≤ 0.05 for comparing the survival curves in each group to the untreated group in each of the experiments using ANOVA with Bonferroni multiple comparisons tests, two-tailed. B. Effect on mean body weights. *, P value < 0.05 for comparing body weights (g, mean ± standard deviation) in each group to the untreated group using the Student's t-Test, two-tailed.
4. Discussion
Despite the effectiveness of influenza virus vaccines, there is an annual need to predict the next year's viruses to incorporate the best antigens into a vaccine. At the present time the period between selecting the antigens and producing and distributing vaccine is long and some years can impinge upon the start of influenza season. Even with adequate vaccine supply, there are individuals who are not protected or who fail to be vaccinated. For the elderly, influenza can be quite severe and can cause pneumonia and death. Clearly in addition to vaccines, there is a need for antiviral drugs. For pandemic avian influenza viruses antigen predictions for vaccines will be even more difficult (Subbaraco and Joseph, 2007). Until it is known what viral properties will allow an avian influenza virus to be transmitted from human to human, stockpiling avian-specific vaccine(s) is problematic and the topic of much discussion pro and con. There will be a need for antivirals especially in the early phase of transmission.
Currently, there are two classes of FDA-approved drugs available for influenza infections. The adamantanes (amantadine and rimantadine) that bind to the M2 protein of the influenza virus and the neuraminidase inhibitors (oseltamivir and zanamivir) that inhibit the enzymatic activity of the surface glycoprotein, neuraminidase. While the adamantanes are active only against the type A and not the type B influenza viruses, the neuraminidase inhibitors are effective against both types. In clinical settings, influenza viruses have developed resistance to both classes of drugs. The clinical significance of the mutations is not clear, but it has been shown that avian H5N1 viruses with engineered neuraminidase inhibitor resistance retain their replication efficiency and pathogenicity in mice (Yen et al., 2007). With the effectiveness shown here for MegaRibavirin aerosol treatment and the lack of development of drug resistance in the clinical setting, ribavirin aerosol treatment could be an additional drug for influenza A viruses and based on previous clinical efficacy studies (Gilbert et al., 1985; McClung et al., 1983), probably influenza B viruses. A single, daily 30-min aerosol treatment with MegaRibavirin protected mice infected with influenza A/HK8/68 (H3N2) virus with greater than a 90% survival rate if treated for 4 days starting 24 hr after infection or even a 68% survival rate if treatment was delayed for as much as 72 hr and treatment was for only two days.
The efficacy of standard and “high dose” ribavirin aerosols in both animal models (Gilbert and Knight, 1986;Wyde et al., 1986) and in uncomplicated influenza (Gilbert and Knight, 1990) as well as in influenzal pneumonia (Knight and Gilbert, 1988) has been demonstrated previously. In light of the development of resistance to the FDA-approved drugs, the use of MegaRibavirin aerosol treatment alone or in combination with the other drugs for avian influenza needs to be tested. Because of their different modes of action, combinations of rimantadine (Galabov et al., 2006) and amantadine (Ilyushina et al., 2007a) with oseltamivir for oral treatment of mice infected with H3N2 and H5N1 viruses, respectively, have been tested and have shown an advantage over a single drug treatment. Oral ribavirin in combination with oseltamivir for the treatment of recent isolates of human A (H1N1) and B influenza viruses (Smee et al., 2006) and prophylactic treatment of avian H5N1 virus (Ilyushina et al., 2007b) was shown to be better than the drugs alone. Although with influenza A virus, delaying treatment with oseltamivir even one day caused it to be ineffective. With single drug treatments, ribavirin appeared to be more effective than oseltamivir. When choosing a treatment, individuals will have been infected for at least one or two days before symptoms appear and treatment is started. Thus, the best treatment will be with a drug or drugs that have demonstrated effectiveness when administered several days after infection.
MegaRibavirin aerosol treatment of influenza in a lethal mouse model has demonstrated important advantages over the FDA-approved (standard) or even the “high dose” treatment regimens. The short treatment period of only 30 min, once or even twice daily, is much shorter than the standard 12 h or ‘high dose” 2 h, 3-times daily treatments. In a clinical setting, the 30-min treatment period would allow more time for other procedures, require one-third or one-sixth the amount of ribavirin and reduce environmental contamination from the aerosol. Further pharmacokinetic and toxicity studies using the MegaRibavirin regimen need to be performed. From earlier ribavirin aerosol pharmacokinetic studies (Gilbert and Wyde, 1988), distribution of ribavirin in the lungs, blood and other organs differed between the standard and “high dose” formulations. Ribavirin was found to reach high concentrations in the lungs and then be distributed at lower concentrations to the blood and other organs including the brain. The fact that ribavirin aerosol treatment will deliver drug to the lungs, stomach, blood and brain may have particular importance in avian influenza if it behaves in man as it does in birds with spread from the lungs to intestines and brain unlike normal human influenza virus which is localized to the lungs. Ribavirin aerosol administered for short durations as with influenza at the standard dose was safely delivered and did not produce any of the side effects associated with oral or intravenous ribavirin (Gilbert et al., 1985;Knight et al., 1981;McClung et al., 1983;Wilson et al., 1984). It is not anticipated that the short 30-min aerosol treatment with MegaRibavirin will change the safety profile since the dose delivered is equal to or less than the other previously used aerosol treatment regimens (Table 1).
Ribavirin and ribavirin aerosol have had issues related to safety. One of these has been mutagenicity and the ability of ribavirin to promote error pone replication in the laboratory setting (Chung et al., 2007;Crotty et al., 2000, 2001). The clinical significance of such studies is not clear. Ribavirin is FDA-approved for treatment of respiratory syncytial virus (RSV) and hepatitis C virus (HCV) infections. Thousand of patients have been treated and a ribavirin-resistant virus has not been found. In addition, an analysis of ribavirin mutagenicity in human HCV infected patients revealed that ribavirin monotherapy did not increase the rate of variation of the consensus sequence, the mutation frequency, the error generation rate, or the between-sample genetic distance (Chevaliez et al., 2007). The conclusion was that ribavirin does not act as an HCV mutagen in vivo. The second issue was environmental contamination by ribavirin aerosol and concerns related to potential teratogenicity for women of childbearing age with secondary exposure to aerosolized ribavirin. Since the early ribavirin aerosol studies were conducted, many steps can and have been implemented to limit secondary ribavirin exposure to anyone (Krilov, 2002). Most notably are the use of containment or scavenger systems and use of shorter treatment protocols (e.g., “high dose”). The MegaRibavirin regimen with its 30-min treatment period and newer equipment would reduce the chance of secondary exposure even more.
In summary, MegaRibavirin aerosol treatment in a lethal influenza mouse model is effective. If these results translate to the clinical setting as in the past, it should prove to be an effective treatment for influenza treatment in general. In addition from results of oral ribavirin studies by others, it should be useful as an aerosol treatment of pandemic avian influenza where short treatments once or twice daily starting two or more days after infection will be advantageous. Combination of ribavirin aerosol and other drug treatments (e.g., oseltamivir) should be considered.
Acknowledgements
This work was supported by contract N01-AI-15437 from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boeckh M, Englund J, Li Y, Miller C, Cross A, Fernandez H, Kuypers J, Kim H, Gnann J, Whitley R, NIAID Collaborative Antiviral Study Group Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin. Infect. Dis. 2007;44:245–249. doi: 10.1086/509930. [DOI] [PubMed] [Google Scholar]
- Chevaliez S, Brillet R, Lazaro E, Hezode C, Pawlotsky J-M. Analysis of ribavirin mutagenicity in human hepatitis C virus infection. J. Virol. 2007;81:7732–7741. doi: 10.1128/JVI.00382-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DH, Sun Y, Parker W, Arterburn J, Bartolucci A, Jonsson CB. Ribavirin reveals a lethal threshold of allowable mutation frequency for hantaan virus. J. Virol. 2007 doi: 10.1128/JVI.00874-07. Epub ahead of print 2007 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Cameron CE, Andino R. RNA virus error catastrophe: Direct molecular test by using ribavirin. Proc. Natl. Acad. Sci. USA. 2001;98:6895–6900. doi: 10.1073/pnas.111085598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Maag D, Arnold JJ, Zhong WD, Lau JYN, Hong Z, Andino R, Cameron CE. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nature Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- Englund JA, Piedra PA, Ahn Y-M, Gilbert BE, Hiatt P. High-dose, short-duration ribavirin aerosol therapy compared with standard ribavirin therapy in children with suspected respiratory syncytial virus infection. J. Pediatr. 1994;125:635–641. doi: 10.1016/s0022-3476(94)70026-5. [DOI] [PubMed] [Google Scholar]
- Englund JA, Piedra PA, Jefferson LS, Wilson SZ, Taber LH, Gilbert BE. High-dose, short-duration ribavirin aerosol therapy in children with suspected respiratory syncytial virus infection. J. Pediatr. 1990;117:313–320. doi: 10.1016/s0022-3476(05)80554-2. [DOI] [PubMed] [Google Scholar]
- Galabov AS, Simeonova L, Gegova G. Rimantadine and oseltamivir demonstrate synergistic combination effect in an experimental infection with type A (H3N2) influenza virus in mice. Antivir. Chem. Chemother. 2006;17:251–258. doi: 10.1177/095632020601700502. [DOI] [PubMed] [Google Scholar]
- Gilbert BE, Knight V. Biochemistry and clinical applications of ribavirin. Antimicrob. Agents Chemother. 1986;30:201–205. doi: 10.1128/aac.30.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert BE, Knight V. Ribavirin aerosol as the treatment for influenza. Drugs Today. 1990;26:195–205. [Google Scholar]
- Gilbert BE, Wilson SZ, Knight V, Couch RB, Quarles JM, Dure L, Hayes N, Willis G. Ribavirin small-particle aerosol treatment of infections caused by influenza virus strains A/Victoria/7/83 (H1N1) and B/Texas/1/84. Antimicrob. Agents Chemother. 1985;27:309–313. doi: 10.1128/aac.27.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert BE, Wyde PR. Pharmacokinetics of ribavirin aerosol in mice. Antimicrob. Agents Chemother. 1988;32:117–121. doi: 10.1128/aac.32.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert BE, Wyde PR, Ambrose MW, Wilson SZ, Knight V. Further studies with short duration ribavirin aerosol for the treatment of influenza virus infection in mice and respiratory syncytial virus infection in cotton rats. Antiviral Res. 1992;17:33–42. doi: 10.1016/0166-3542(92)90088-m. [DOI] [PubMed] [Google Scholar]
- Gilbert BE, Wyde PR, Wilson SZ, Robins RK. Aerosol and intraperitoneal administration of ribavirin and ribavirin triacetate: Pharmacokinetics and protection of mice against intracerebral infection with influenza A/WSN virus. Antimicrob. Agents Chemother. 1991;35:1448–1453. doi: 10.1128/aac.35.7.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono AT, Johnson BA, Grgurich WF, Youssef JG, Corcoran TE, Seiler DA, Dauber JH, Smaldone GC, Zeevi A, Yousem SA, Fung JJ, Burckart GJ, McCurry KR, Griffith BP. A randomized trial of inhaled cyclosporine in lung-transplant recipients. N. Engl. J. Med. 2006;354:141–150. doi: 10.1056/NEJMoa043204. [DOI] [PubMed] [Google Scholar]
- Ilyushina NA, Hoffmann E, Solomon R, Webster RG, Govorkova EA. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antiviral Ther. 2007a;12:363–370. [PubMed] [Google Scholar]
- Ilyushina NA, Webster RG, Govorkova EA. Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antiviral Res. 2007b;74:A31–A32. doi: 10.1128/AAC.01579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight V, Gilbert B. Antiviral therapy with small particle aerosols. Eur. J. Clin. Microbiol. Infect. Dis. 1988;7:721–731. doi: 10.1007/BF01975037. [DOI] [PubMed] [Google Scholar]
- Knight V, Gilbert BE. Ribavirin aerosol treatment of influenza. In: Knight V, Gilbert BE, editors. Infectious disease clinics of North America volume 1: Antiviral chemotherapy. W.B. Saunders Co.; Philadelphia: 1987. pp. 441–457. [PubMed] [Google Scholar]
- Knight V, Koshkina NV, Waldrep JC, Giovanella BC, Gilbert BE. Anti-cancer effect of 9-nitrocamptothecin liposome aerosol on human cancer xenografts in nude mice. Cancer Chemother. Pharmacol. 1999;44:177–186. doi: 10.1007/s002800050965. [DOI] [PubMed] [Google Scholar]
- Knight V, McClung HW, Wilson SZ, Waters BK, Quarles JM, Cameron RW, Greggs SE, Zerwas JM, Couch RB. Ribavirin small-particle aerosol treatment of influenza. Lancet. 1981;2(8253):945–949. doi: 10.1016/s0140-6736(81)91152-1. [DOI] [PubMed] [Google Scholar]
- Knight V, Yu CP, Gilbert BE, Divine GW. Estimating the dosage of ribavirin aerosol according to age and other variables. J. Infect. Dis. 1988;158:443–448. doi: 10.1093/infdis/158.2.443. [DOI] [PubMed] [Google Scholar]
- Koshkina NV, Golunski E, Roberts LE, Gilbert BE, Knight V. Cyclosporin A aerosol improves the anticancer effect of paclitaxel aerosol in mice. J. Aerosol Med. 2004;17:7–14. doi: 10.1089/089426804322994415. [DOI] [PubMed] [Google Scholar]
- Krilov LR. Safety issues related to the administration of ribavirin. Pediatr. Infect. Dis. J. 2002;21:479–481. doi: 10.1097/00006454-200205000-00037. [DOI] [PubMed] [Google Scholar]
- McClung HW, Knight V, Gilbert BE, Wilson SZ, Quarles JM, Divine GW. Ribavirin aerosol treatment of influenza B virus infection. J. Amer. Med. Assoc. 1983;249:2671–2674. [PubMed] [Google Scholar]
- Phalen RF. Inhalation studies: foundations and techniques. CRC Press, Inc.; Boca Raton: 1984. [Google Scholar]
- Schlesinger RB. Comparative deposition of inhaled aerosols in experimental animals and humans: A review. J. Toxicol. Environ. Health. 1985;15:197–214. doi: 10.1080/15287398509530647. [DOI] [PubMed] [Google Scholar]
- Smee DF, Wong M-H, Bailey KW, Sidwell RW. Activities of oseltamivir and ribavirin used alone and in combination against infections in mice with recent isolates of influenza A (H1N1) and B viruses. Antivir. Chem. Chemother. 2006;17:185–192. doi: 10.1177/095632020601700403. [DOI] [PubMed] [Google Scholar]
- Subbaraco K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat. Rev. Immunol. 2007;7:267–278. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber LH, Knight V, Gilbert B,E, McClung HW, Wilson SZ, Norton HJ, Thurston JM, Gordon WH, Atmar RL, Schlaudt WR. Ribavirin aerosol treatment of bronchiolitis associated with respiratory syncytial virus infection in infants. Pediatrics. 1983;72:613–618. [PubMed] [Google Scholar]
- Verschraegen CF, Gilbert BE, Loyer E, Huaringa A, Walsh G, Newman RA, Knight V. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(S)-camptothecin in patients with advanced pulmonary malignancies. Clin. Cancer Res. 2004;10:2319–2326. doi: 10.1158/1078-0432.ccr-0929-3. [DOI] [PubMed] [Google Scholar]
- Whimbey E, Champlin RE, Englund JA, Mirza NQ, Piedra PA, Goodrich JM, Przepiorka D, Luna MA, Morice RC, Neumann JL, Elting LS, Bodey GP. Combination therapy with aerosolized ribavirin and intravenous immunoglobulin for respiratory syncytial virus disease in adult bone marrow transplant recipients. Bone Marrow Transplant. 1995;16:393–399. [PubMed] [Google Scholar]
- Wiggins NA. The development of a mathematical approximation technique to determine the mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) of drug particles in an inhalation aerosol spray. Drug Develop. Ind. Pharm. 1991;17:1971–1986. [Google Scholar]
- Wilson SZ, Gilbert BE, Quarles JM, Knight V, McClung HW, Moore RV, Couch RB. Treatment of influenza A(H1N1) virus infection with ribavirin aerosol. Antimicrob. Agents Chemother. 1984;26:200–203. doi: 10.1128/aac.26.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde PR, Couch RB, Mackler BF, Cate TR, Levy BM. Effects of low- and high-passage influenza virus infection in normal and nude mice. Infect. Immun. 1977;15:221–229. doi: 10.1128/iai.15.1.221-229.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde PR, Wilson SZ, Gilbert BE, Smith RHA. Protection of mice from lethal influenza virus infection with high dose-short duration ribavirin aerosol. Antimicrob. Agents Chemother. 1986;30:942–944. doi: 10.1128/aac.30.6.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde PR, Wilson SZ, Petrella R, Gilbert BE. Efficacy of high dose-short duration ribavirin aerosol in the treatment of respiratory syncytial virus infected cotton rats and influenza B virus infected mice. Antiviral Res. 1987;7:211–220. doi: 10.1016/0166-3542(87)90029-5. [DOI] [PubMed] [Google Scholar]
- Yen HL, Ilyushina NA, Salomon R, Hoffmann E, Webster RG, Govorkova EA. Neuraminidase inhibitor resistant recombinant A/Vietnan/1203/04 (H5N1) influenza viruses retain their replication efficiency and pathogenicity in vitro and in vivo. J. Virol. 2007 doi: 10.1128/JVI.01067-07. Epub ahead of print 2007 Sep12. [DOI] [PMC free article] [PubMed] [Google Scholar]