Abstract
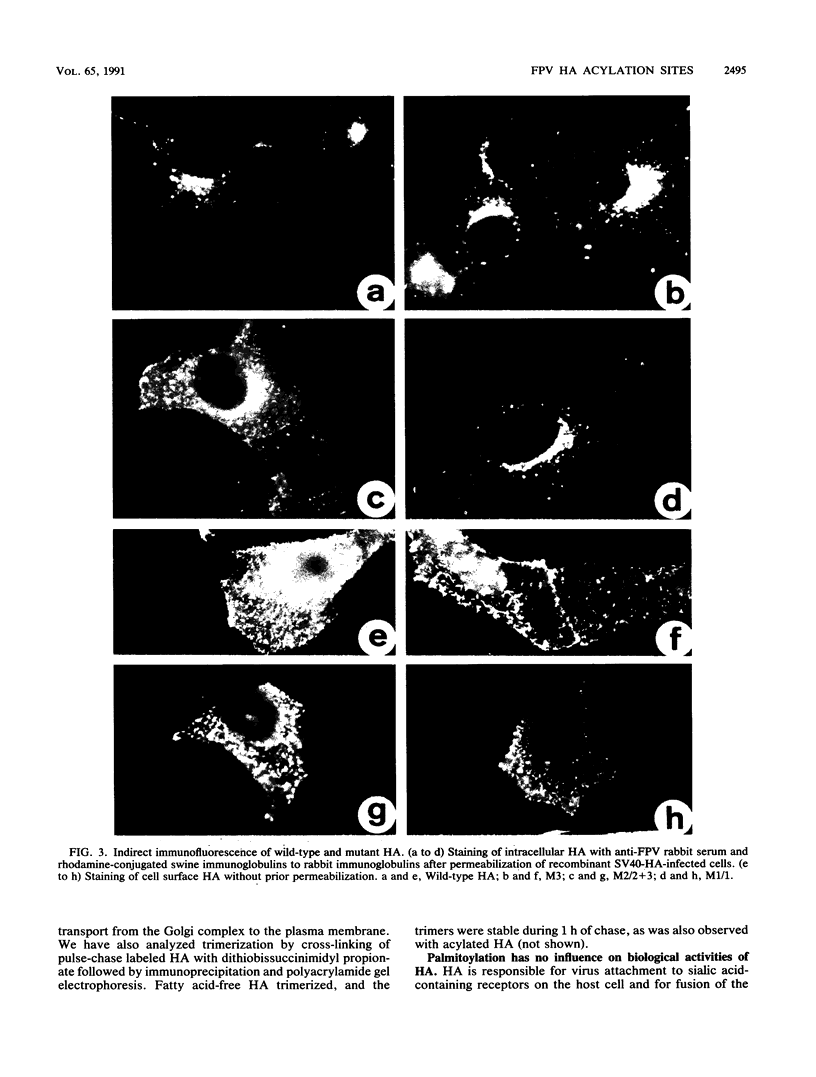
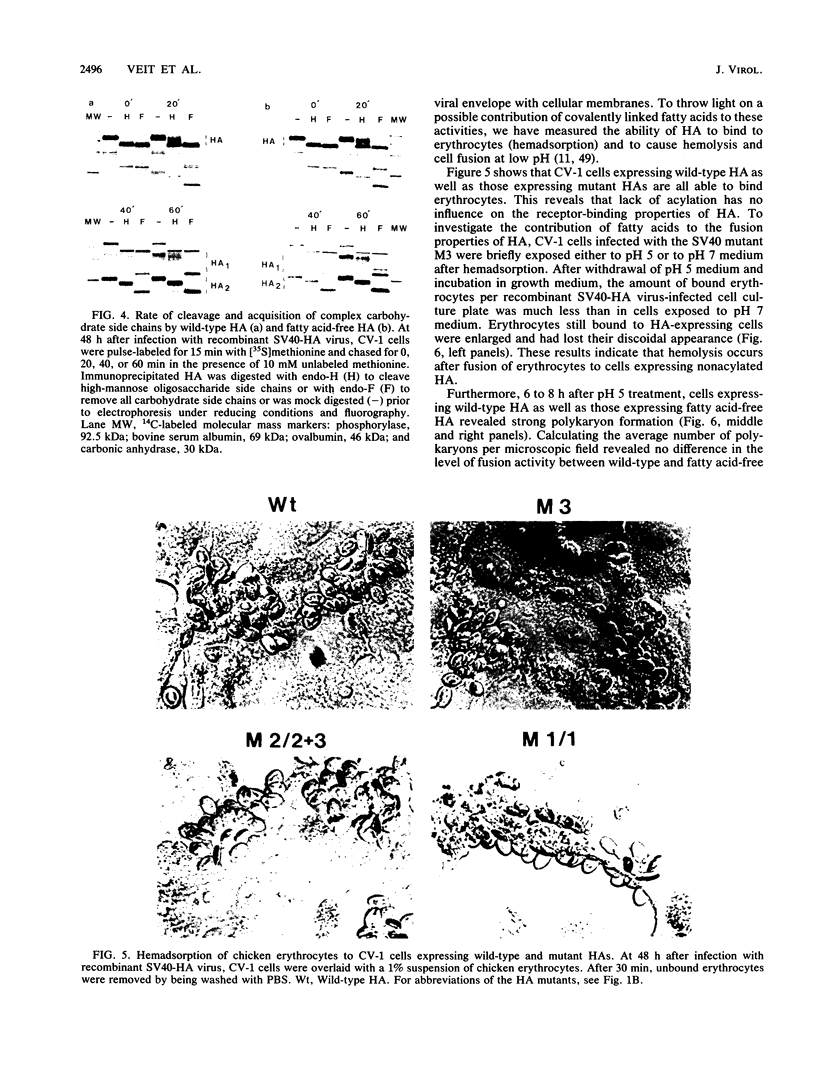
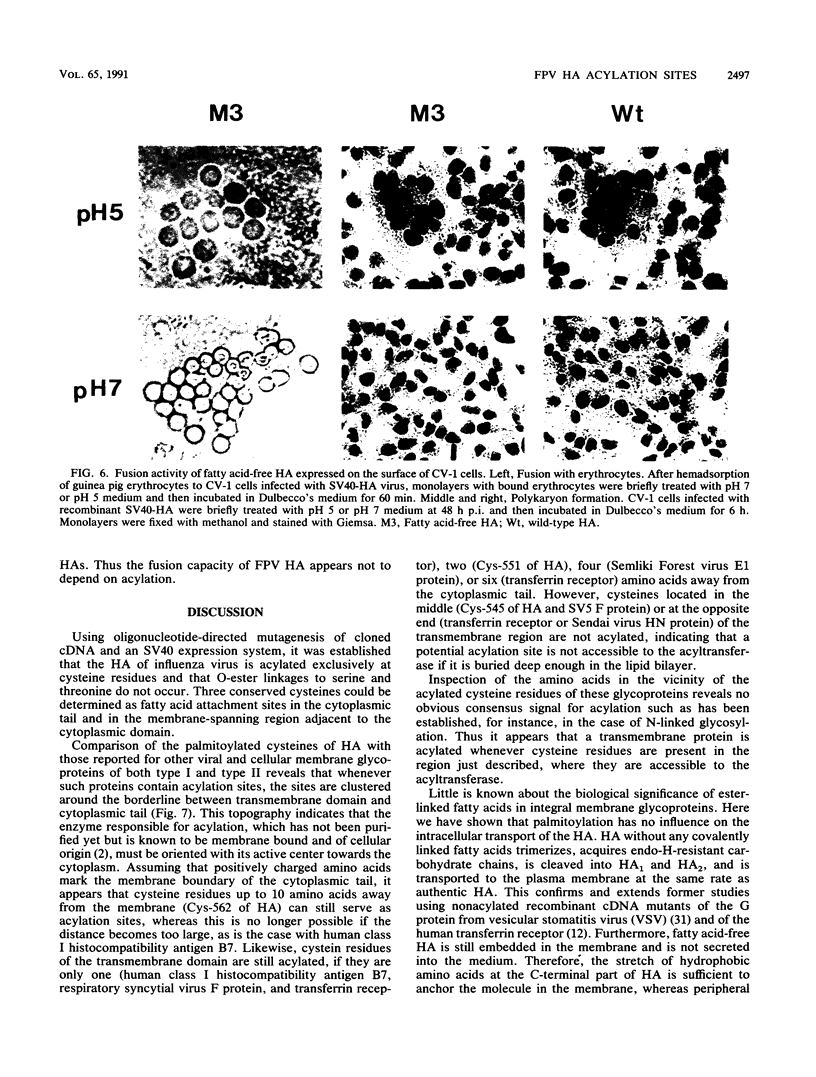
The hemagglutinin (HA) of influenza virus is a type I transmembrane glycoprotein which is acylated with long-chain fatty acids. In this study we have used oligonucleotide-directed mutagenesis of cloned cDNA and a simian virus 40 expression system to determine the fatty acid binding site in HA and to examine possible functions of covalently linked fatty acids. The results show that the HA is acylated through thioester linkages at three highly conserved cysteine residues located in the cytoplasmic domain and at the carboxy-terminal end of the transmembrane region, whereas a cysteine located in the middle of the membrane-spanning domain is not acylated. Mutants lacking fatty acids at individual or all three attachment sites acquire endoglycosidase H-resistant oligosaccharide side chains, are cleaved into HA1 and HA2 subunits, and are transported to the plasma membrane at rates similar to that of wild-type HA. All mutants are membrane bound and not secreted into the medium. These results exclude transport signal and membrane-anchoring functions of covalently linked fatty acids for this integral membrane glycoprotein. Furthermore, lack of acylation has no obvious influence on the biological activities of HA: cells expressing fatty acid-free HA bind to and, after brief exposure to mildly acidic pH, fuse with erythrocytes; the HA-induced polykaryon formation is not impaired, either. Other possible functions of covalently linked fatty acids in integral membrane glycoproteins which cannot be examined in conventional cDNA expression systems are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arumugham R. G., Seid R. C., Jr, Doyle S., Hildreth S. W., Paradiso P. R. Fatty acid acylation of the fusion glycoprotein of human respiratory syncytial virus. J Biol Chem. 1989 Jun 25;264(18):10339–10342. [PubMed] [Google Scholar]
- Berger M., Schmidt M. F. Cell-free fatty acid acylation of Semliki Forest viral polypeptides with microsomal membranes from eukaryotic cells. J Biol Chem. 1984 Jun 10;259(11):7245–7252. [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C., Roth M. G., Sambrook J., Gething M. J. Mutations in the cytoplasmic domain of the influenza virus hemagglutinin affect different stages of intracellular transport. J Cell Biol. 1985 Mar;100(3):704–714. doi: 10.1083/jcb.100.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami M., Luytjes W., Krystal M., Palese P. Introduction of site-specific mutations into the genome of influenza virus. Proc Natl Acad Sci U S A. 1990 May;87(10):3802–3805. doi: 10.1073/pnas.87.10.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H., Kretzschmar E., Klingeborn B., Rott R., Klenk H. D., Garten W. The structure of serotype H10 hemagglutinin of influenza A virus: comparison of an apathogenic avian and a mammalian strain pathogenic for mink. Virology. 1988 Aug;165(2):428–437. doi: 10.1016/0042-6822(88)90586-7. [DOI] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Grand R. J. Acylation of viral and eukaryotic proteins. Biochem J. 1989 Mar 15;258(3):625–638. doi: 10.1042/bj2580625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R. T., Rott R., Klenk H. D. Influenza viruses cause hemolysis and fusion of cells. Virology. 1981 Apr 15;110(1):243–247. doi: 10.1016/0042-6822(81)90030-1. [DOI] [PubMed] [Google Scholar]
- Jing S. Q., Trowbridge I. S. Identification of the intermolecular disulfide bonds of the human transferrin receptor and its lipid-attachment site. EMBO J. 1987 Feb;6(2):327–331. doi: 10.1002/j.1460-2075.1987.tb04758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil W., Geyer R., Dabrowski J., Dabrowski U., Niemann H., Stirm S., Klenk H. D. Carbohydrates of influenza virus. Structural elucidation of the individual glycans of the FPV hemagglutinin by two-dimensional 1H n.m.r. and methylation analysis. EMBO J. 1985 Oct;4(10):2711–2720. doi: 10.1002/j.1460-2075.1985.tb03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R. The molecular biology of influenza virus pathogenicity. Adv Virus Res. 1988;34:247–281. doi: 10.1016/S0065-3527(08)60520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J. The HLA-D-associated invariant chain binds palmitic acid at the cysteine adjacent to the membrane segment. J Biol Chem. 1986 Mar 5;261(7):3434–3440. [PubMed] [Google Scholar]
- Kuroda K., Hauser C., Rott R., Klenk H. D., Doerfler W. Expression of the influenza virus haemagglutinin in insect cells by a baculovirus vector. EMBO J. 1986 Jun;5(6):1359–1365. doi: 10.1002/j.1460-2075.1986.tb04367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B., Schmidt M. F. Membrane fusion induced by influenza virus hemagglutinin requires protein bound fatty acids. FEBS Lett. 1986 Jun 23;202(1):127–132. doi: 10.1016/0014-5793(86)80662-7. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Marc D., Drugeon G., Haenni A. L., Girard M., van der Werf S. Role of myristoylation of poliovirus capsid protein VP4 as determined by site-directed mutagenesis of its N-terminal sequence. EMBO J. 1989 Sep;8(9):2661–2668. doi: 10.1002/j.1460-2075.1989.tb08406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeve C. W., Williams D. Fatty acids on the A/Japan/305/57 influenza virus hemagglutinin have a role in membrane fusion. EMBO J. 1990 Dec;9(12):3857–3866. doi: 10.1002/j.1460-2075.1990.tb07604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd B. F., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem. 1989 May 5;264(13):7564–7569. [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Bogachuk A. S. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988 Mar 28;230(1-2):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Harris T. J., Lamb R. A. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6706–6710. doi: 10.1073/pnas.81.21.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. A., Jr, Pal R., Barenholz Y., Wagner R. R. Fluorescence anisotropy of a fatty acid covalently linked in vivo to the glycoprotein of vesicular stomatitis virus. J Biol Chem. 1981 Mar 25;256(6):2625–2627. [PubMed] [Google Scholar]
- Pfanner N., Orci L., Glick B. S., Amherdt M., Arden S. R., Malhotra V., Rothman J. E. Fatty acyl-coenzyme A is required for budding of transport vesicles from Golgi cisternae. Cell. 1989 Oct 6;59(1):95–102. doi: 10.1016/0092-8674(89)90872-6. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Adams G. A., Gallione C. J. The presence of cysteine in the cytoplasmic domain of the vesicular stomatitis virus glycoprotein is required for palmitate addition. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2050–2054. doi: 10.1073/pnas.81.7.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Malfer C. Cerulenin blocks fatty acid acylation of glycoproteins and inhibits vesicular stomatitis and Sindbis virus particle formation. J Biol Chem. 1982 Sep 10;257(17):9887–9890. [PubMed] [Google Scholar]
- Schmidt M. F. Acylation of viral spike glycoproteins: a feature of enveloped RNA viruses. Virology. 1982 Jan 15;116(1):327–338. doi: 10.1016/0042-6822(82)90424-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F. Fatty acylation of proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):411–426. doi: 10.1016/0304-4157(89)90013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Lambrecht B. On the structure of the acyl linkage and the function of fatty acyl chains in the influenza virus haemagglutinin and the glycoproteins of Semliki Forest virus. J Gen Virol. 1985 Dec;66(Pt 12):2635–2647. doi: 10.1099/0022-1317-66-12-2635. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F. The transfer of myristic and other fatty acids on lipid and viral protein acceptors in cultured cells infected with Semliki Forest and influenza virus. EMBO J. 1984 Oct;3(10):2295–2300. doi: 10.1002/j.1460-2075.1984.tb02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Schmidt M. F., Rott R. Chemical identification of cysteine as palmitoylation site in a transmembrane protein (Semliki Forest virus E1). J Biol Chem. 1988 Dec 15;263(35):18635–18639. [PubMed] [Google Scholar]
- Schuy W., Will C., Kuroda K., Scholtissek C., Garten W., Klenk H. D. Mutations blocking the transport of the influenza virus hemagglutinin between the rough endoplasmic reticulum and the Golgi apparatus. EMBO J. 1986 Nov;5(11):2831–2836. doi: 10.1002/j.1460-2075.1986.tb04576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Iwasaki K., Shibuta H. Determination of the complete nucleotide sequence of the Sendai virus genome RNA and the predicted amino acid sequences of the F, HN and L proteins. Nucleic Acids Res. 1986 Feb 25;14(4):1545–1563. doi: 10.1093/nar/14.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H., Virág I. Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43. J Cell Biol. 1989 Feb;108(2):613–624. doi: 10.1083/jcb.108.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., Hillen H., Schröder W., Deutzmann R. The primary structure of bovine brain myelin lipophilin (proteolipid apoprotein). Hoppe Seylers Z Physiol Chem. 1983 Oct;364(10):1455–1466. doi: 10.1515/bchm2.1983.364.2.1455. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Veit M., Herrler G., Schmidt M. F., Rott R., Klenk H. D. The hemagglutinating glycoproteins of influenza B and C viruses are acylated with different fatty acids. Virology. 1990 Aug;177(2):807–811. doi: 10.1016/0042-6822(90)90554-5. [DOI] [PubMed] [Google Scholar]
- Veit M., Schmidt M. F., Rott R. Different palmitoylation of paramyxovirus glycoproteins. Virology. 1989 Jan;168(1):173–176. doi: 10.1016/0042-6822(89)90417-0. [DOI] [PubMed] [Google Scholar]
- Waterfield M., Scrace G., Skehel J. Disulphide bonds of haemagglutinin of Asian influenza virus. Nature. 1981 Jan 29;289(5796):422–424. doi: 10.1038/289422a0. [DOI] [PubMed] [Google Scholar]
- White J., Helenius A., Gething M. J. Haemagglutinin of influenza virus expressed from a cloned gene promotes membrane fusion. Nature. 1982 Dec 16;300(5893):658–659. doi: 10.1038/300658a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Snider M. D., Porter M., Lodish H. F. Mutants of vesicular stomatitis virus blocked at different stages in maturation of the viral glycoprotein. Cell. 1980 Sep;21(2):417–427. doi: 10.1016/0092-8674(80)90478-x. [DOI] [PubMed] [Google Scholar]