Abstract
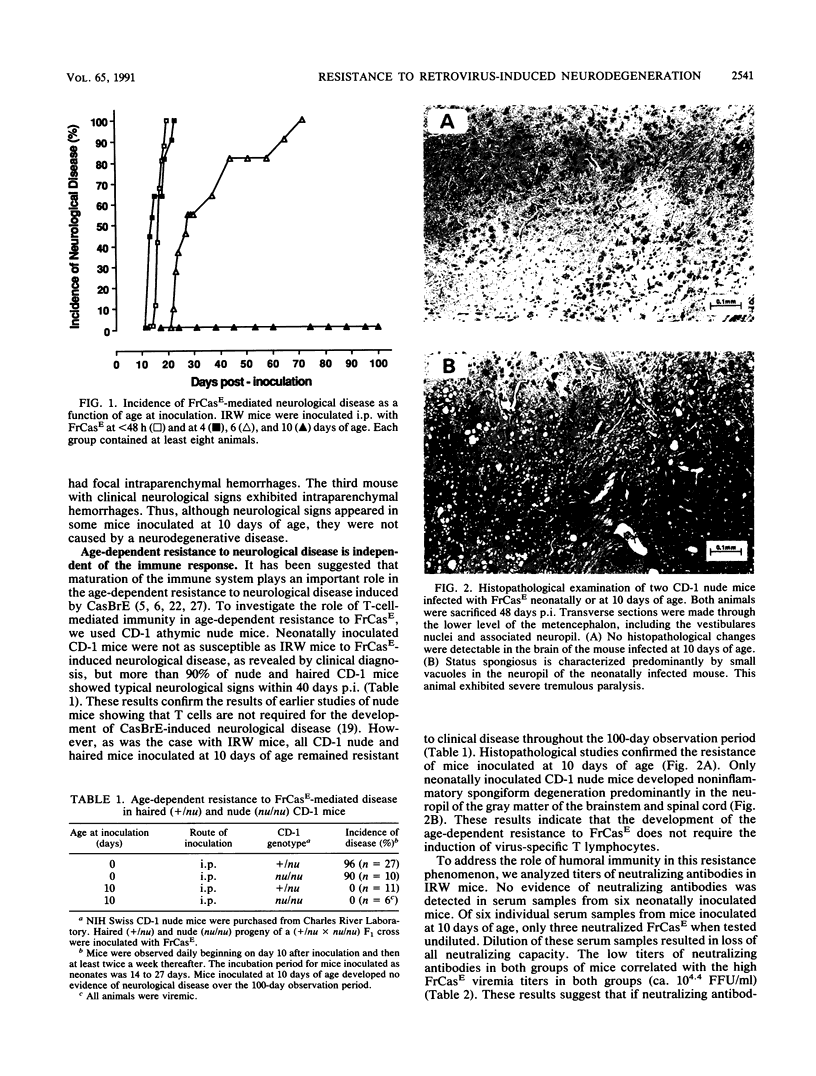
The murine retrovirus CasBrE causes a noninflammatory spongiform degeneration of the central nervous system (CNS). Mice inoculated as neonates develop viremia and are susceptible to disease. However, mice inoculated at 10 days of age do not develop viremia and are totally resistant to the neurologic disease. We recently described a highly neurovirulent chimeric virus, FrCasE (J. L. Portis, S. Czub, C. F. Garon, and F. J. McAtee, J. Virol. 64:1648-1656, 1990), which contains the env gene of CasBrE. Mice inoculated at 10 days of age with this virus developed a viremia comparable to that in neonatally inoculated mice but, surprisingly, were still completely resistant to the neurodegenerative disease. A comparison of the tissue distribution of virus replication for mice inoculated at 1 or 10 days of age was determined by Southern blot analysis for the quantification of viral DNA and by infectious-center assay for the quantification of virus-producing cells. The levels of virus replication in the spleens were comparable in the two groups. In contrast, virus replication in the CNS of the resistant 10-day-old mice was markedly restricted (100- to 1,000-fold). Intracerebral inoculation did not overcome this restriction. A similar pattern of CNS-specific restriction of virus replication and resistance to disease was observed in athymic NIH Swiss nude mice inoculated at 10 days of age, suggesting that T-cell immunity was not involved. From our results, we conclude that the age-dependent resistance to disease is a consequence of the restriction of virus replication within the CNS due to the developmental state of the organ.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. M., Gardner M. B. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J Neuropathol Exp Neurol. 1974 Apr;33(2):285–307. doi: 10.1097/00005072-197404000-00007. [DOI] [PubMed] [Google Scholar]
- Brown P. O. Integration of retroviral DNA. Curr Top Microbiol Immunol. 1990;157:19–48. doi: 10.1007/978-3-642-75218-6_2. [DOI] [PubMed] [Google Scholar]
- Ewert D. L., Steiner I., DuHadaway J. In ovo infection with the avian retrovirus RAV-1 leads to persistent infection of the central nervous system. Lab Invest. 1990 Feb;62(2):156–162. [PubMed] [Google Scholar]
- Fujita S. Quantitative analysis of cell proliferation and differentiation in the cortex of the postnatal mouse cerebellum. J Cell Biol. 1967 Feb;32(2):277–287. doi: 10.1083/jcb.32.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Robbins D. S., Morse H. C., 3rd Role of immunity in age-related resistance to paralysis after murine leukemia virus infection. J Virol. 1984 Dec;52(3):734–738. doi: 10.1128/jvi.52.3.734-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Ruscetti S. K., Morse H. C., 3rd Pathogenesis of paralysis and lymphoma associated with a wild mouse retrovirus infection. Part 1. Age- and dose-related effects in susceptible laboratory mice. J Neuroimmunol. 1981 Sep;1(3):275–285. doi: 10.1016/0165-5728(81)90031-x. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T., McFarland H. F., Levy S. E. Age-dependent resistance to viral encephalitis: studies of infections due to Sindbis virus in mice. J Infect Dis. 1972 Mar;125(3):257–262. doi: 10.1093/infdis/125.3.257. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Nicolaiew N., DesGroseillers L., Rassart E. Molecular cloning of infectious viral DNA from ecotropic neurotropic wild mouse retrovirus. J Virol. 1983 Mar;45(3):1159–1163. doi: 10.1128/jvi.45.3.1159-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNIN C. M. Virus-tissue union and the pathogenesis of enterovirus infections. J Immunol. 1962 May;88:556–569. [PubMed] [Google Scholar]
- Kai K., Furuta T. Isolation of paralysis-inducing murine leukemia viruses from Friend virus passaged in rats. J Virol. 1984 Jun;50(3):970–973. doi: 10.1128/jvi.50.3.970-973.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Spontaneous hepatitis and cerebellar "hypoplasia" in suckling rats due to congenital infections with rat virus. Am J Pathol. 1966 Sep;49(3):457–475. [PMC free article] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Viral etiology of spontaneous ataxia of cats. Am J Pathol. 1966 Jun;48(6):991–1011. [PMC free article] [PubMed] [Google Scholar]
- McAtee F. J., Portis J. L. Monoclonal antibodies specific for wild mouse neurotropic retrovirus: detection of comparable levels of virus replication in mouse strains susceptible and resistant to paralytic disease. J Virol. 1985 Dec;56(3):1018–1022. doi: 10.1128/jvi.56.3.1018-1022.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVERMAN J. R., KILHAM L. The interrelation of age. immune response, and susceptibility to mumps virus in hamsters. J Immunol. 1953 Nov;71(5):352–358. [PubMed] [Google Scholar]
- Officer J. E., Tecson N., Estes J. D., Fontanilla E., Rongey R. W., Gardner M. B. Isolation of a neurotropic type C virus. Science. 1973 Sep 7;181(4103):945–947. doi: 10.1126/science.181.4103.945. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Lampert P. W., Lee S., Dixon F. J. Pathogenesis of the slow disease of the central nervous system associated with WM 1504 E virus. I. Relationship of strain susceptibility and replication to disease. Am J Pathol. 1977 Jul;88(1):193–212. [PMC free article] [PubMed] [Google Scholar]
- Pickel K., Müller M. A., ter Meulen V. Influence of the immune system on the course of infection with murine coronavirus JHM in suckling mice. Adv Exp Med Biol. 1981;142:375–386. doi: 10.1007/978-1-4757-0456-3_31. [DOI] [PubMed] [Google Scholar]
- Portis J. L., Czub S., Garon C. F., McAtee F. J. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J Virol. 1990 Apr;64(4):1648–1656. doi: 10.1128/jvi.64.4.1648-1656.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J., Hayes S. F. Horizontal transmission of murine retroviruses. J Virol. 1987 Apr;61(4):1037–1044. doi: 10.1128/jvi.61.4.1037-1044.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L. Wild mouse retrovirus: pathogenesis. Curr Top Microbiol Immunol. 1990;160:11–27. doi: 10.1007/978-3-642-75267-4_2. [DOI] [PubMed] [Google Scholar]
- Prasad G., Stoica G., Wong P. K. The role of the thymus in the pathogenesis of hind-limb paralysis induced by ts1, a mutant of Moloney murine leukemia virus-TB. Virology. 1989 Apr;169(2):332–340. doi: 10.1016/0042-6822(89)90158-x. [DOI] [PubMed] [Google Scholar]
- Reinarz A. B., Broome M. G., Sagik B. P. Age-dependent resistance of mice to sindbis virus infection: viral replication as a function of host age. Infect Immun. 1971 Feb;3(2):268–273. doi: 10.1128/iai.3.2.268-273.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D. S., Hoffman P. M. Virus-specific cytotoxic lymphocyte response in a neurotropic murine leukemia virus infection. J Neuroimmunol. 1991 Jan;31(1):9–17. doi: 10.1016/0165-5728(91)90081-h. [DOI] [PubMed] [Google Scholar]
- SIGEL M. M. Influence of age on susceptibility to virus infections with particular reference to laboratory animals. Annu Rev Microbiol. 1952;6:247–280. doi: 10.1146/annurev.mi.06.100152.001335. [DOI] [PubMed] [Google Scholar]
- Sitbon M., Sola B., Evans L., Nishio J., Hayes S. F., Nathanson K., Garon C. F., Chesebro B. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell. 1986 Dec 26;47(6):851–859. doi: 10.1016/0092-8674(86)90800-7. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Padgett T., Heasley S., Simon G., Bishop J. M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977 Jun;11(2):307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]





