Abstract
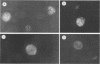
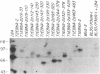
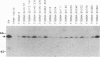
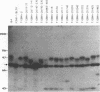
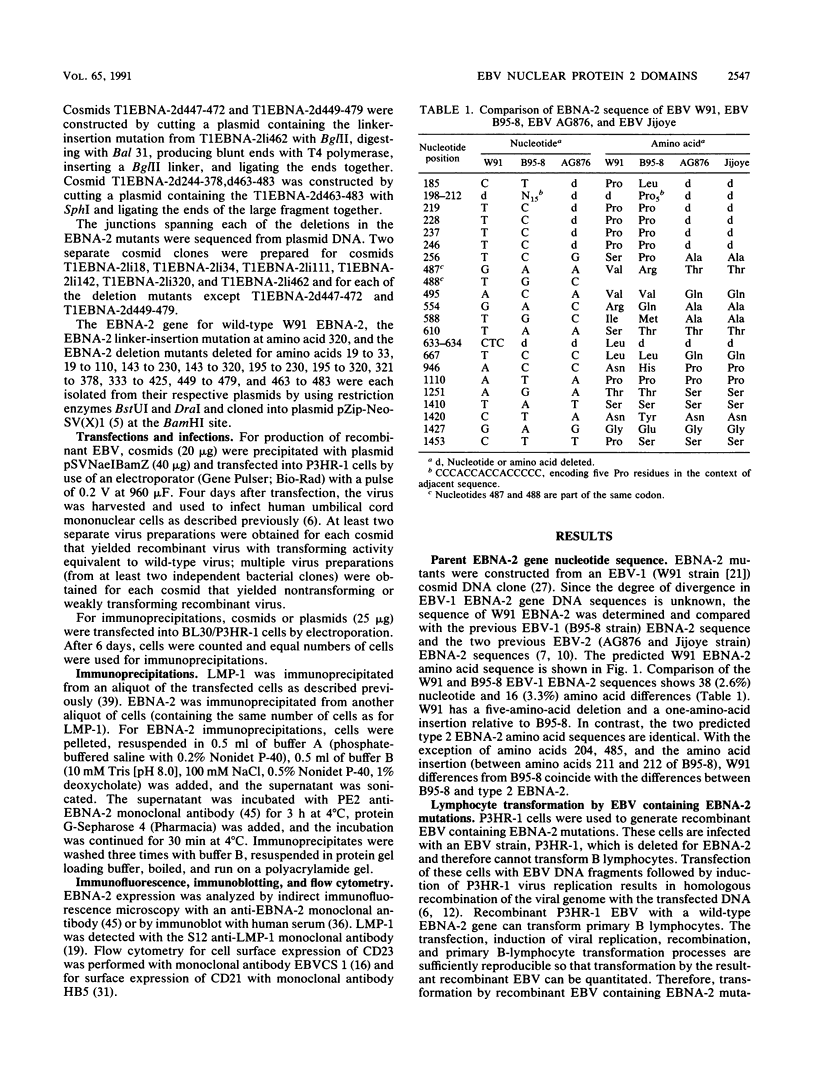
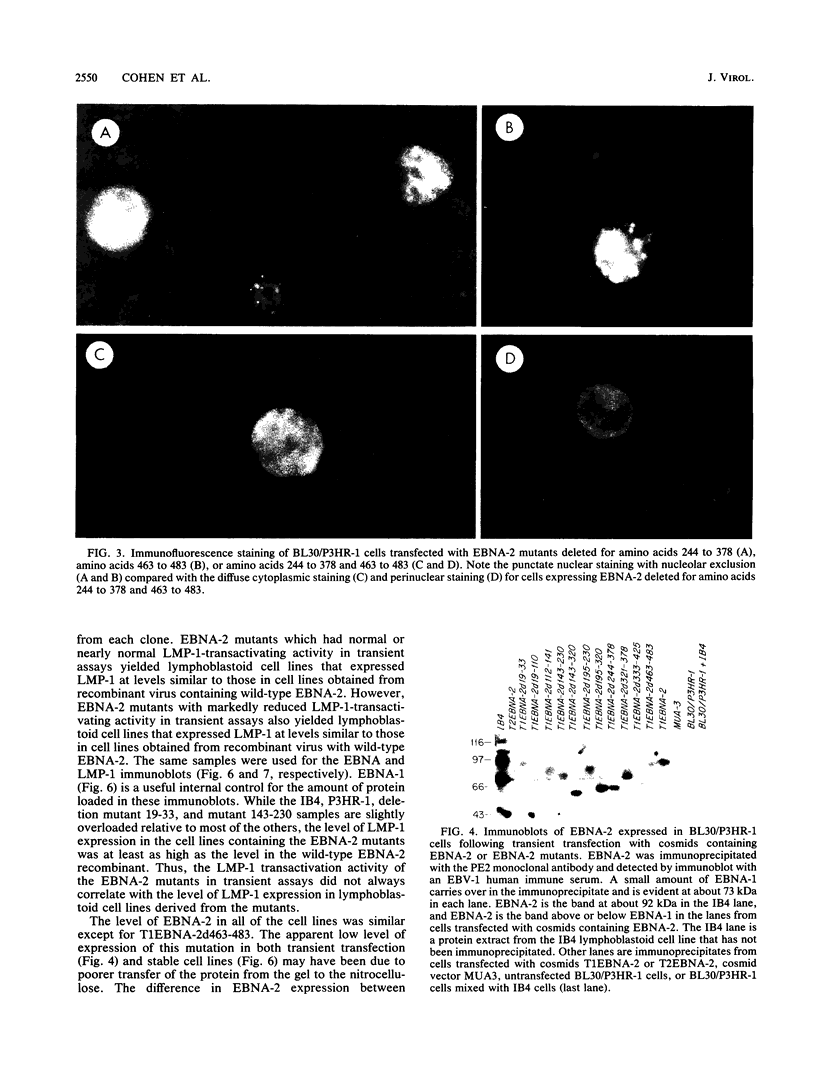
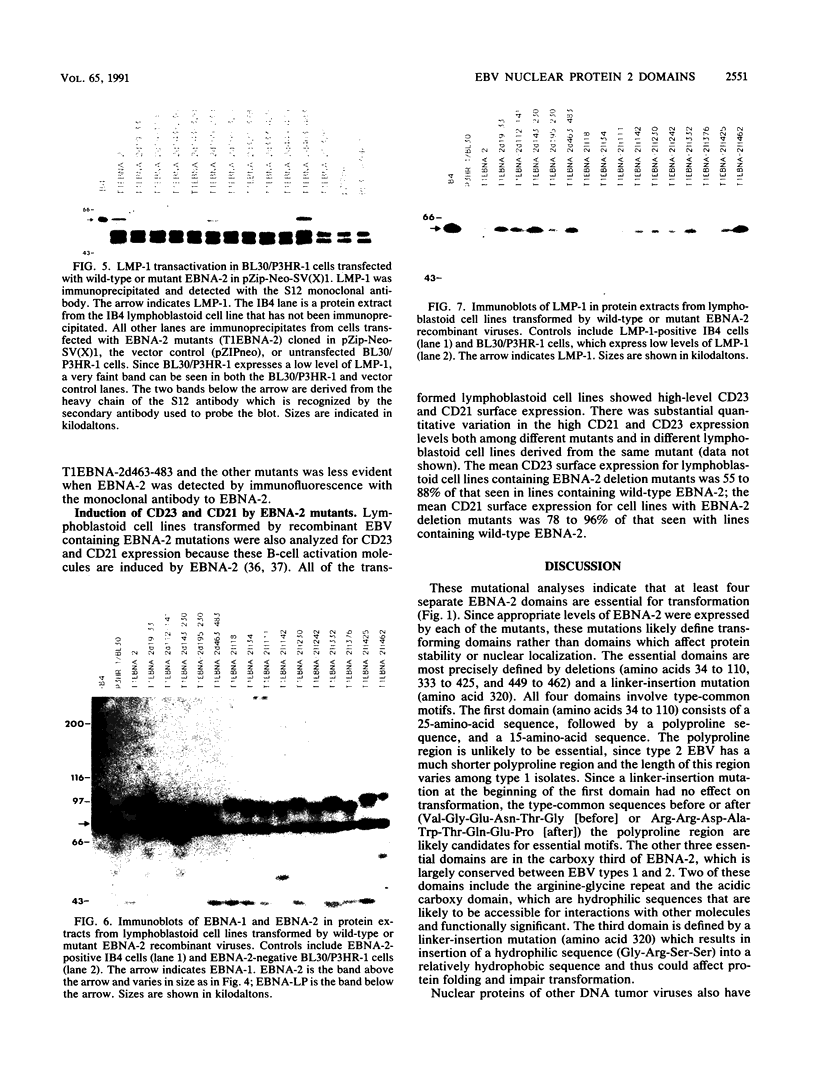
Epstein-Barr virus (EBV) nuclear protein 2 (EBNA-2) is essential for B-lymphocyte growth transformation. EBNA-2 transactivates expression of the EBV latent membrane protein (LMP-1) and also transactivates expression of the B-lymphocyte proteins CD21 and CD23. In order to analyze the functional domains of EBNA-2, we constructed 11 linker-insertion and 15 deletion mutations. Each of the mutant EBNA-2 proteins localized to the nucleus, and each was expressed at levels similar to wild-type EBNA-2. Deletion of both EBNA-2 basic domains was required to prevent nuclear localization, indicating that either is sufficient for nuclear translocation. The mutant EBNA-2 genes were assayed for lymphocyte transformation after recombination with the EBNA-2-deleted P3HR-1 EBV genome and for LMP-1 transactivation following transfection into P3HR-1-infected B-lymphoma cells. Cell lines transformed by recombinant EBV carrying EBNA-2 mutations were assayed for growth properties and LMP-1, CD21, and CD23 expression. The mutational analysis indicates that at least four separate EBNA-2 domains are essential for lymphocyte transformation. Two other domains are necessary for the full transforming phenotype. Two deletion and eight linker-insertion mutations did not reduce transforming activity. Mutations which diminish or abolish lymphocyte transformation also diminish or abolish LMP-1 transactivation, respectively. Cells transformed by recombinant EBV carrying EBNA-2 genes with diminished or normal transforming activity all expressed high levels of LMP-1, CD23, and CD21. These findings suggest that transactivation of these viral and cellular genes by EBNA-2 plays a critical role in lymphocyte transformation by EBV. Furthermore, these results indicate that the transformation and transactivation functions of EBNA-2 may not be separable.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbot S. D., Rowe M., Cadwallader K., Ricksten A., Gordon J., Wang F., Rymo L., Rickinson A. B. Epstein-Barr virus nuclear antigen 2 induces expression of the virus-encoded latent membrane protein. J Virol. 1990 May;64(5):2126–2134. doi: 10.1128/jvi.64.5.2126-2134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auborn K., Guo M., Prives C. Helicase, DNA-binding, and immunological properties of replication-defective simian virus 40 mutant T antigens. J Virol. 1989 Feb;63(2):912–918. doi: 10.1128/jvi.63.2.912-918.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baichwal V. R., Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988 May;2(5):461–467. [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Mannick J., Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Hennessy K., Chamnankit L., Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Wang F., Hennessy K., Woodland E., Rickinson A., Kieff E. Expression of the Epstein-Barr virus nuclear protein 2 in rodent cells. J Virol. 1986 Aug;59(2):453–462. doi: 10.1128/jvi.59.2.453-462.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Dillner J., Kallin B. The Epstein-Barr virus proteins. Adv Cancer Res. 1988;50:95–158. doi: 10.1016/s0065-230x(08)60436-4. [DOI] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989 Aug 3;340(6232):393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Smith A. E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984 Nov;139(1):109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- Kintner C., Sugden B. Identification of antigenic determinants unique to the surfaces of cells transformed by Epstein-Barr virus. Nature. 1981 Dec 3;294(5840):458–460. doi: 10.1038/294458a0. [DOI] [PubMed] [Google Scholar]
- Knutson J. C. The level of c-fgr RNA is increased by EBNA-2, an Epstein-Barr virus gene required for B-cell immortalization. J Virol. 1990 Jun;64(6):2530–2536. doi: 10.1128/jvi.64.6.2530-2536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Kieff E. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J Virol. 1990 May;64(5):2319–2326. doi: 10.1128/jvi.64.5.2319-2326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. P., Staunton D., Thorley-Lawson D. A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985 Sep;55(3):710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Coope D., Niederman J., Pagano J. Biological properties and viral surface antigens of Burkitt lymphoma- and mononucleosis- derived strains of Epstein-Barr virus released from transformed marmoset cells. J Virol. 1976 Jun;18(3):1071–1080. doi: 10.1128/jvi.18.3.1071-1080.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Shope T., Coope D., Waters L., Pagano J., Bornkamn G., Henle W. Lymphoma in cotton-top marmosets after inoculation with Epstein-Barr virus: tumor incidence, histologic spectrum antibody responses, demonstration of viral DNA, and characterization of viruses. J Exp Med. 1977 Apr 1;145(4):948–967. doi: 10.1084/jem.145.4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Kurilla M. G., Griffin H. M., Brooks J. M., Mackett M., Arrand J. R., Rowe M., Burrows S. R., Moss D. J., Kieff E. Human cytotoxic T-cell responses against Epstein-Barr virus nuclear antigens demonstrated by using recombinant vaccinia viruses. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2906–2910. doi: 10.1073/pnas.87.8.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., Sample C., Kieff E. Subnuclear localization and phosphorylation of Epstein-Barr virus latent infection nuclear proteins. Virology. 1990 Jun;176(2):563–574. doi: 10.1016/0042-6822(90)90027-o. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N., Dambaugh T., Kieff E. DNA of Epstein-Barr virus VIII: B95-8, the previous prototype, is an unusual deletion derivative. Cell. 1980 Nov;22(1 Pt 1):257–267. doi: 10.1016/0092-8674(80)90173-7. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Young L. S., Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J Virol. 1987 May;61(5):1310–1317. doi: 10.1128/jvi.61.5.1310-1317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A., Peden K. W., Pipas J. M. The large tumor antigen of simian virus 40 encodes at least two distinct transforming functions. J Virol. 1989 Dec;63(12):5459–5463. doi: 10.1128/jvi.63.12.5459-5463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. L., Kalderon D., Smith A. E., Tevethia M. J. Dissociation of Rb-binding and anchorage-independent growth from immortalization and tumorigenicity using SV40 mutants producing N-terminally truncated large T antigens. Virology. 1990 Sep;178(1):15–34. doi: 10.1016/0042-6822(90)90375-2. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. The truncated form of the Epstein-Barr virus latent-infection membrane protein expressed in virus replication does not transform rodent fibroblasts. J Virol. 1988 Jul;62(7):2337–2346. doi: 10.1128/jvi.62.7.2337-2346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Petti L., Braun D., Seung S., Kieff E. A bicistronic Epstein-Barr virus mRNA encodes two nuclear proteins in latently infected, growth-transformed lymphocytes. J Virol. 1987 Apr;61(4):945–954. doi: 10.1128/jvi.61.4.945-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Tsang S. F., Kurilla M. G., Cohen J. I., Kieff E. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J Virol. 1990 Jul;64(7):3407–3416. doi: 10.1128/jvi.64.7.3407-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Kanda T., Sato H., Furuno A., Yoshiike K. Mutational analysis of human papillomavirus type 16 E7 functions. J Virol. 1990 Jan;64(1):207–214. doi: 10.1128/jvi.64.1.207-214.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Ruley H. E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988 Jan;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L., Alfieri C., Hennessy K., Evans H., O'Hara C., Anderson K. C., Ritz J., Shapiro R. S., Rickinson A., Kieff E. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989 Oct 19;321(16):1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]