Abstract
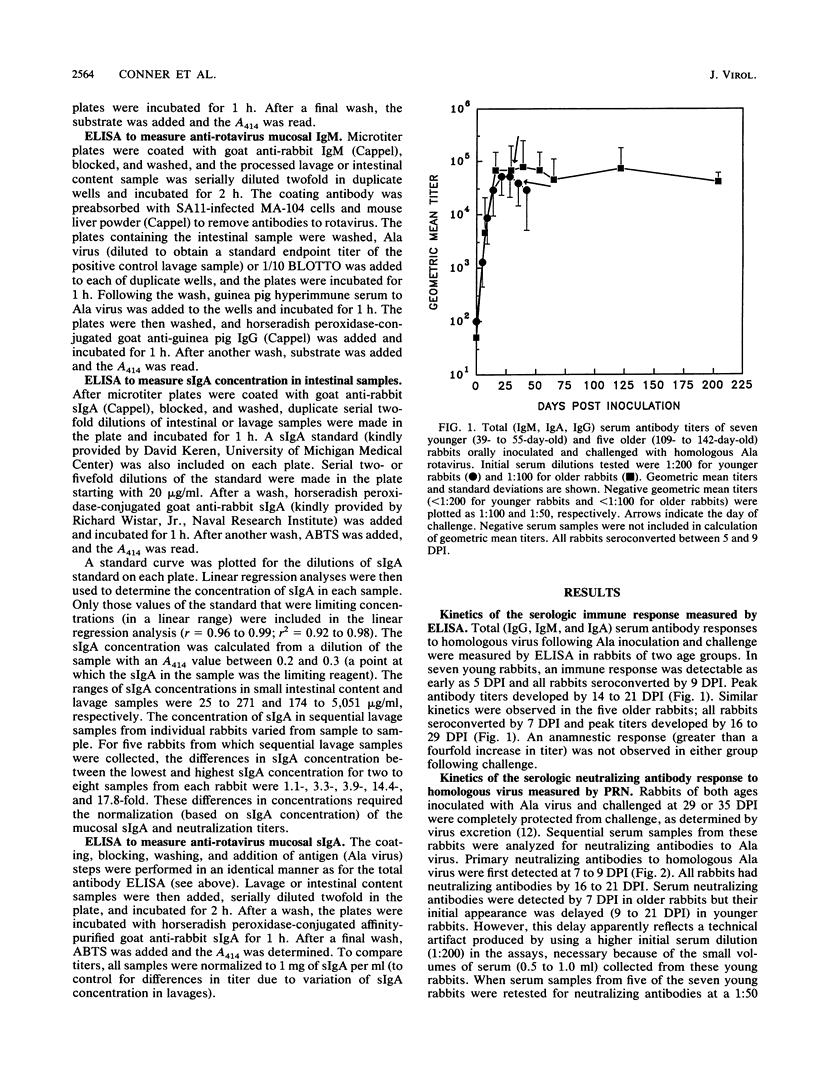
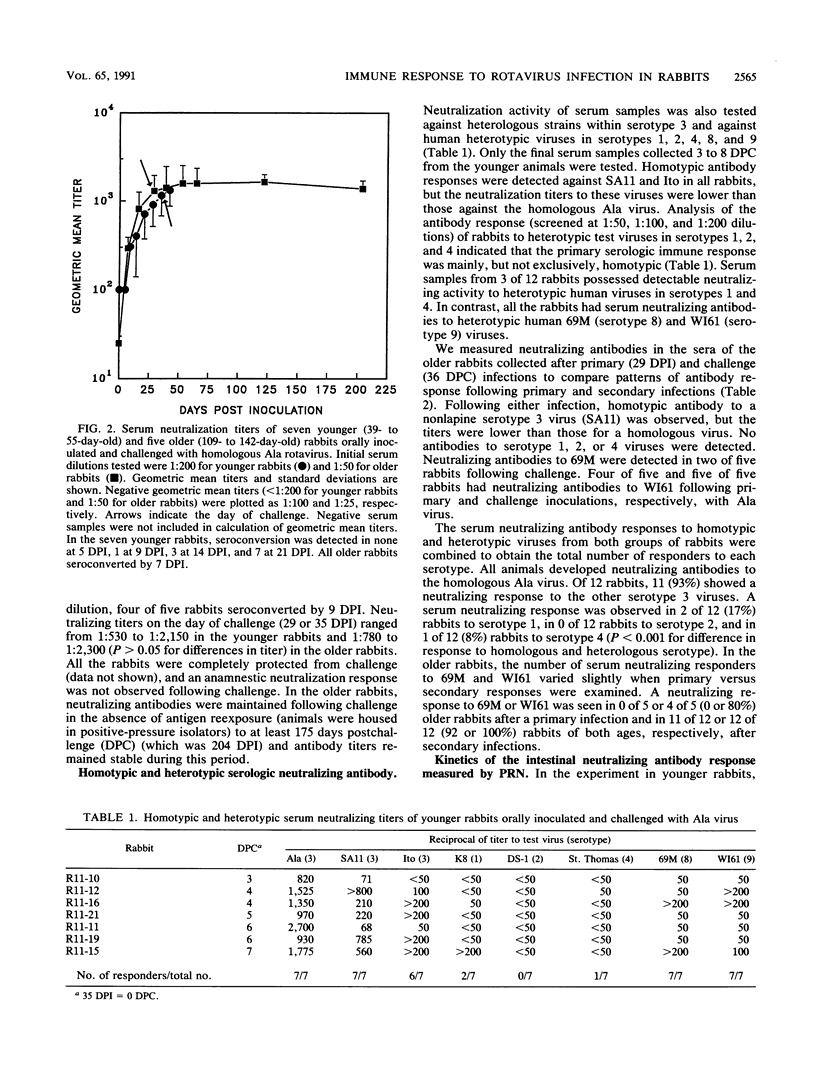
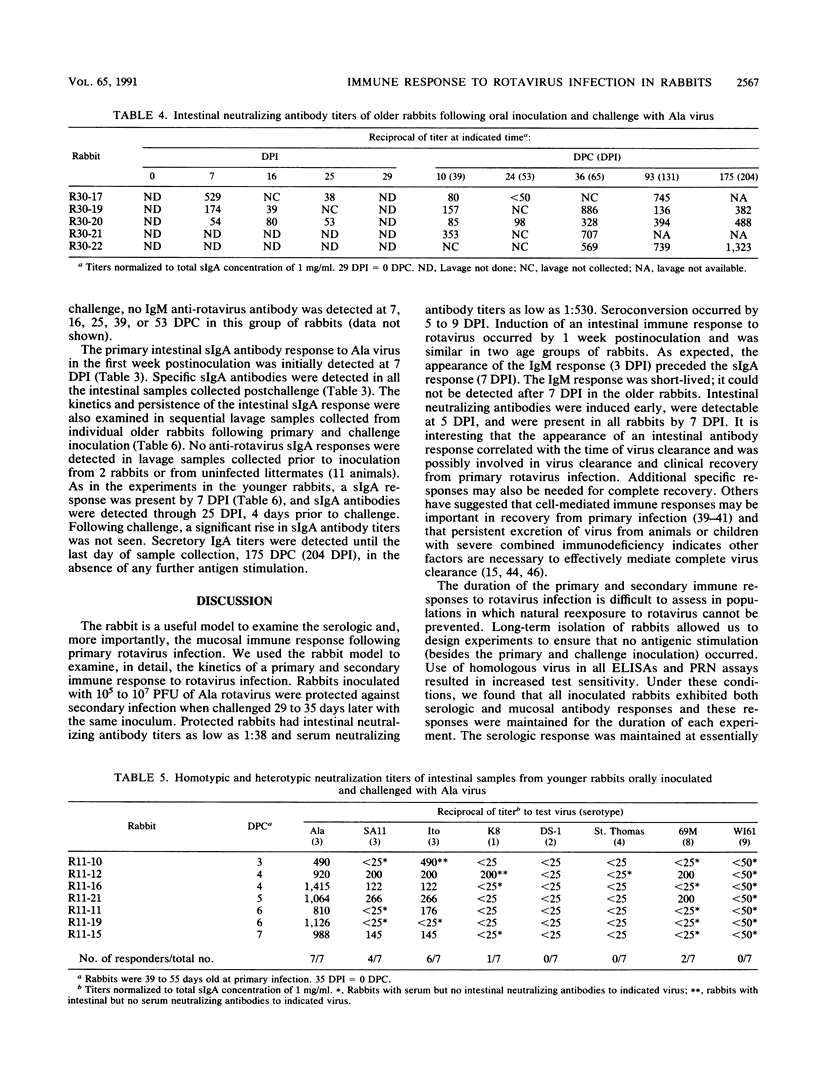
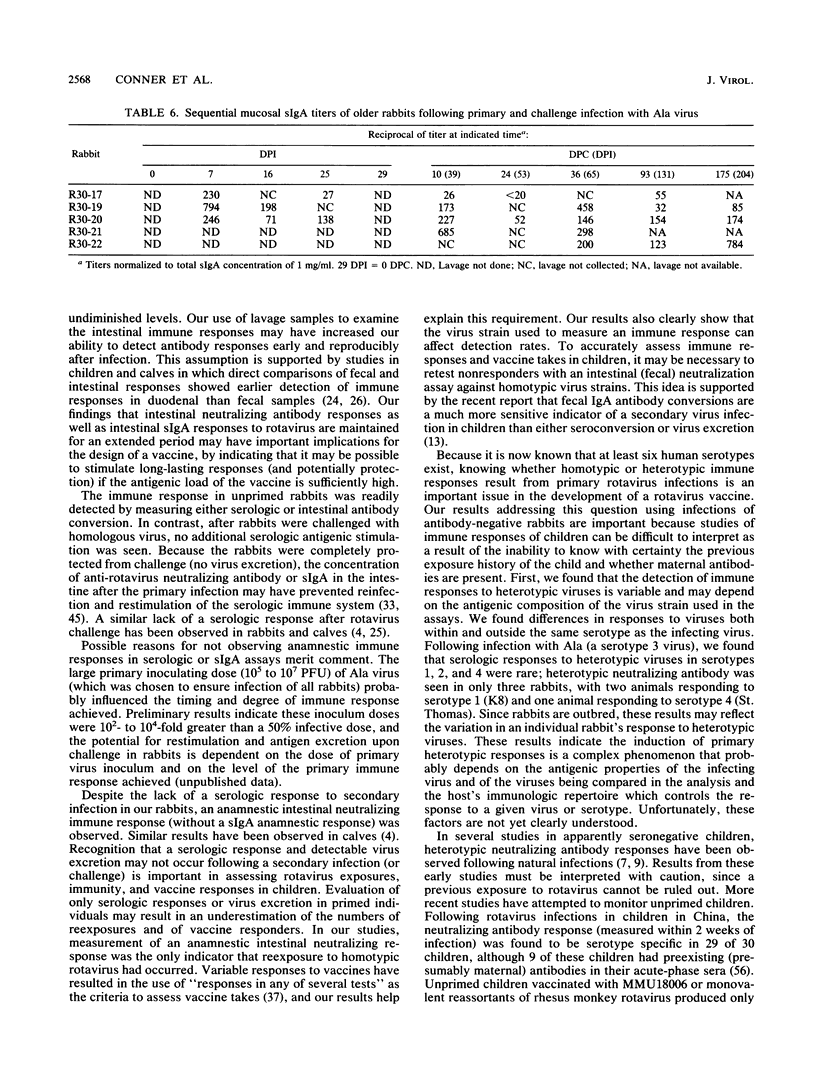
We examined the humoral immune response to rotavirus infection in specific pathogen-free rabbits inoculated and challenged orally with rabbit Ala rotavirus (7.5 x 10(5) to 1 x 10(7) PFU). The humoral immune response in both serologic and mucosal samples was monitored by using total antibody enzyme-linked immunosorbent assays (ELISAs), isotype-specific ELISAs, and plaque reduction neutralization assays. Following a primary infection, all rabbits shed virus and serologic and mucosal antibody responses were initially detected by 1 week postinoculation. Intestinal immunoglobulin M was detected by 3 days postinoculation, and secretory immunoglobulin A was detected by 6 days postinoculation. Following challenge, rabbits were protected (no detectable virus shedding) from infection. An anamnestic immune response was observed only with mucosal neutralizing antibodies, and all serologic and mucosal immune responses persisted at high levels until at least 175 days postchallenge (204 days postinoculation). Detection of neutralization responses was influenced by the virus strain used in the neutralization assay; all inoculated rabbits developed detectable serum and intestinal neutralizing antibodies against the infecting (Ala) virus strain. Neutralization activity in both serum and mucosal samples was generally, but not exclusively, homotypic (VP7 serotype 3) after both primary and challenge inoculations with Ala virus. Heterotypic serum neutralization activity was observed with serotype 8 (9 of 12 rabbits) and 9 (12 of 12 rabbits) viruses and may be based on reactivity with the outer capsid protein VP4 or on a shared epitope in the C region of VP7. Comparisons of heterologous (serotype 3) and heterotypic neutralizing responses in mucosal and serologic samples revealed that 43% (21 of 49) of the responses were discordant. In 19 of 49 (39%) of these cases, a heterotypic serologic response was seen in the absence of a heterotypic mucosal response, but in 2 of 49 (4%) instances, a heterotypic mucosal response was seen in the absence of a concomitant serologic response. These results provide insight into factors which may affect detection of heterotypic responses.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein D. I., Ziegler J. M., Ward R. L. Rotavirus fecal IgA antibody response in adults challenged with human rotavirus. J Med Virol. 1986 Dec;20(4):297–304. doi: 10.1002/jmv.1890200402. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Tzipori S. R., Coulson B. S., Unicomb L. E., Albert M. J., Barnes G. L. Heterologous protection against rotavirus-induced disease in gnotobiotic piglets. J Clin Microbiol. 1986 Dec;24(6):1023–1028. doi: 10.1128/jcm.24.6.1023-1028.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C., Oldham G. Avirulent rotavirus infections protect calves from disease with and without inducing high levels of neutralizing antibody. J Gen Virol. 1987 Sep;68(Pt 9):2311–2317. doi: 10.1099/0022-1317-68-9-2311. [DOI] [PubMed] [Google Scholar]
- Burr D. H., Kerner D. T., Blanco C. S., Bourgeois A. L., Wistar R., Jr Gastric lavage: a simple method to obtain IgA-rich intestinal secretions from the rabbit. J Immunol Methods. 1987 May 20;99(2):277–281. doi: 10.1016/0022-1759(87)90138-4. [DOI] [PubMed] [Google Scholar]
- Cadranel S., Zeglache S., Jonckheer T., Zissis G., Andre F. E., Bogaerts H., Delem A. Factors affecting antibody response of newborns to repeated administrations of the rotavirus vaccine RIT 4237. J Pediatr Gastroenterol Nutr. 1987 Jul-Aug;6(4):525–528. doi: 10.1097/00005176-198707000-00005. [DOI] [PubMed] [Google Scholar]
- Chiba S., Yokoyama T., Nakata S., Morita Y., Urasawa T., Taniguchi K., Urasawa S., Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986 Aug 23;2(8504):417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- Christy C., Madore H. P., Treaner J. J., Pray K., Kapikian A. Z., Chanock R. M., Dolin R. Safety and immunogenicity of live attenuated rhesus monkey rotavirus vaccine. J Infect Dis. 1986 Dec;154(6):1045–1047. doi: 10.1093/infdis/154.6.1045. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Dolan K. T., Horton-Slight P., Palmer J., Plotkin S. A. Diverse serologic response to rotavirus infection of infants in a single epidemic. Pediatr Infect Dis. 1985 Nov-Dec;4(6):626–631. doi: 10.1097/00006454-198511000-00006. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Furukawa T., Bell L. M., Offit P. A., Perrella P. A., Plotkin S. A. Immune response of infants and children to low-passage bovine rotavirus (strain WC3). Am J Dis Child. 1986 Apr;140(4):350–356. doi: 10.1001/archpedi.1986.02140180084030. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Offit P. A., Dolan K. T., Tezza A., Gogalin K., Twist E. M., Plotkin S. A. Response of adult human volunteers to oral administration of bovine and bovine/human reassortant rotaviruses. Vaccine. 1986 Mar;4(1):25–31. doi: 10.1016/0264-410x(86)90094-0. [DOI] [PubMed] [Google Scholar]
- Conner M. E., Estes M. K., Graham D. Y. Rabbit model of rotavirus infection. J Virol. 1988 May;62(5):1625–1633. doi: 10.1128/jvi.62.5.1625-1633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Grimwood K., Masendycz P. J., Lund J. S., Mermelstein N., Bishop R. F., Barnes G. L. Comparison of rotavirus immunoglobulin A coproconversion with other indices of rotavirus infection in a longitudinal study in childhood. J Clin Microbiol. 1990 Jun;28(6):1367–1374. doi: 10.1128/jcm.28.6.1367-1374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G. P., Hogg R. J., Kirubakaran C. P. Serum and intestinal immune response to rotavirus enteritis in children. Infect Immun. 1983 May;40(2):447–452. doi: 10.1128/iai.40.2.447-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharakul T., Rott L., Greenberg H. B. Recovery from chronic rotavirus infection in mice with severe combined immunodeficiency: virus clearance mediated by adoptive transfer of immune CD8+ T lymphocytes. J Virol. 1990 Sep;64(9):4375–4382. doi: 10.1128/jvi.64.9.4375-4382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. H., Graham D. Y., Estes M. K. Detection of rotaviruses by nucleic acid hybridization with cloned DNA of simian rotavirus SA11 genes. J Infect Dis. 1985 Aug;152(2):293–300. doi: 10.1093/infdis/152.2.293. [DOI] [PubMed] [Google Scholar]
- Edelman R., Flores J., Kapikian A. Z. Immunity to rotaviruses. Curr Top Microbiol Immunol. 1989;146:123–136. doi: 10.1007/978-3-642-74529-4_14. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Conner M. E., Gilger M. A., Graham D. Y. Molecular biology and immunology of rotavirus infections. Immunol Invest. 1989 Jan-May;18(1-4):571–581. doi: 10.3109/08820138909112264. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y. Identification of rotaviruses of different origins by the plaque-reduction test. Am J Vet Res. 1980 Jan;41(1):151–152. [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Blanco M., Vilar M., Garcia D., Perez M., Daoud N., Midthun K., Kapikian A. Z. Reactions to and antigenicity of two human-rhesus rotavirus reassortant vaccine candidates of serotypes 1 and 2 in Venezuelan infants. J Clin Microbiol. 1989 Mar;27(3):512–518. doi: 10.1128/jcm.27.3.512-518.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. Y., Hoshino Y., Ikegami N. Sequence analysis of the gene encoding the serotype-specific glycoprotein (VP7) of two new human rotavirus serotypes. Virology. 1989 Feb;168(2):429–433. doi: 10.1016/0042-6822(89)90289-4. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Taniguchi K., Mackow E. R., Kapikian A. Z. Homotypic and heterotypic epitope-specific antibody responses in adult and infant rotavirus vaccinees: implications for vaccine development. J Infect Dis. 1990 Apr;161(4):667–679. doi: 10.1093/infdis/161.4.667. [DOI] [PubMed] [Google Scholar]
- Grimwood K., Lund J. C., Coulson B. S., Hudson I. L., Bishop R. F., Barnes G. L. Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol. 1988 Apr;26(4):732–738. doi: 10.1128/jcm.26.4.732-738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambraeus B. A., Hambraeus L. E., Wadell G. Animal model of rotavirus infection in rabbits--protection obtained without shedding of viral antigen. Arch Virol. 1989;107(3-4):237–251. doi: 10.1007/BF01317920. [DOI] [PubMed] [Google Scholar]
- Hjelt K., Grauballe P. C., Andersen L., Schiøtz P. O., Howitz P., Krasilnikoff P. A. Antibody response in serum and intestine in children up to six months after a naturally acquired rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1986 Jan;5(1):74–80. doi: 10.1097/00005176-198601000-00014. [DOI] [PubMed] [Google Scholar]
- Hjelt K., Grauballe P. C., Paerregaard A., Nielsen O. H., Krasilnikoff P. A. Protective effect of preexisting rotavirus-specific immunoglobulin A against naturally acquired rotavirus infection in children. J Med Virol. 1987 Jan;21(1):39–47. doi: 10.1002/jmv.1890210106. [DOI] [PubMed] [Google Scholar]
- Hjelt K., Grauballe P. C., Schiøtz P. O., Andersen L., Krasilnikoff P. A. Intestinal and serum immune response to a naturally acquired rotavirus gastroenteritis in children. J Pediatr Gastroenterol Nutr. 1985 Feb;4(1):60–66. doi: 10.1097/00005176-198502000-00012. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Torres A., Aves T. M., Flores G., Lin J. H., Sereno M. M., Kapikian A. Z. Neutralizing antibody response of gnotobiotic piglets after fetal infection and neonatal challenge with human rotavirus strains. J Infect Dis. 1987 Dec;156(6):1038–1040. doi: 10.1093/infdis/156.6.1038. [DOI] [PubMed] [Google Scholar]
- Hum C. P., Dyall-Smith M. L., Holmes I. H. The VP7 gene of a new G serotype of human rotavirus (B37) is similar to G3 proteins in the antigenic c region. Virology. 1989 May;170(1):55–61. doi: 10.1016/0042-6822(89)90351-6. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Levine M. M., Yolken R. H., VanKirk D. H., Dolin R., Greenberg H. B., Chanock R. M. Oral administration of human rotavirus to volunteers: induction of illness and correlates of resistance. J Infect Dis. 1983 Jan;147(1):95–106. doi: 10.1093/infdis/147.1.95. [DOI] [PubMed] [Google Scholar]
- La Brooy J. T., Davidson G. P., Sherman D. J., Rowley D. The antibody response to bacterial gastroenteritis in serum and secretions. Clin Exp Immunol. 1980 Aug;41(2):290–296. [PMC free article] [PubMed] [Google Scholar]
- Lanata C. F., Black R. E., del Aguila R., Gil A., Verastegui H., Gerna G., Flores J., Kapikian A. Z., Andre F. E. Protection of Peruvian children against rotavirus diarrhea of specific serotypes by one, two, or three doses of the RIT 4237 attenuated bovine rotavirus vaccine. J Infect Dis. 1989 Mar;159(3):452–459. doi: 10.1093/infdis/159.3.452. [DOI] [PubMed] [Google Scholar]
- Losonsky G. A., Rennels M. B., Lim Y., Krall G., Kapikian A. Z., Levine M. M. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatr Infect Dis J. 1988 Jun;7(6):388–393. doi: 10.1097/00006454-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Matsui S. M., Mackow E. R., Greenberg H. B. Molecular determinant of rotavirus neutralization and protection. Adv Virus Res. 1989;36:181–214. doi: 10.1016/s0065-3527(08)60585-0. [DOI] [PubMed] [Google Scholar]
- Midthun K., Pang L. Z., Flores J., Kapikian A. Z. Comparison of immunoglobulin A (IgA), IgG, and IgM enzyme-linked immunosorbent assays, plaque reduction neutralization assay, and complement fixation in detecting seroresponses to rotavirus vaccine candidates. J Clin Microbiol. 1989 Dec;27(12):2799–2804. doi: 10.1128/jcm.27.12.2799-2804.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985 Apr;54(1):58–64. doi: 10.1128/jvi.54.1.58-64.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes appear at the intestinal mucosal surface after rotavirus infection. J Virol. 1989 Aug;63(8):3507–3512. doi: 10.1128/jvi.63.8.3507-3512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes cross-react with target cells infected with different rotavirus serotypes. J Virol. 1988 Jan;62(1):127–131. doi: 10.1128/jvi.62.1.127-131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Greenberg H. B., Dudzik K. I. Rotavirus-specific protein synthesis is not necessary for recognition of infected cells by virus-specific cytotoxic T lymphocytes. J Virol. 1989 Aug;63(8):3279–3283. doi: 10.1128/jvi.63.8.3279-3283.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Noriega L., Arias C. F., López S., Puerto F., Snodgrass D. R., Taniguchi K., Greenberg H. B. Diversity of rotavirus serotypes in Mexican infants with gastroenteritis. J Clin Microbiol. 1990 Jun;28(6):1114–1119. doi: 10.1128/jcm.28.6.1114-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Bogger-Goren S., Li P., Carmody P. J., Barrett H. J., Ogra P. L. Development of serum and intestinal antibody response to rotavirus after naturally acquired rotavirus infection in man. J Med Virol. 1981;8(3):215–222. doi: 10.1002/jmv.1890080309. [DOI] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Dharakul T., Kowalski E., Michalak S., Ogra P. L. Persistent rotavirus infection in mice with severe combined immunodeficiency. J Virol. 1987 Oct;61(10):3345–3348. doi: 10.1128/jvi.61.10.3345-3348.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D., La Brooy J. Intestinal immune responses in relation to diarrhoeal diseases. J Diarrhoeal Dis Res. 1986 Mar;4(1):1–9. [PubMed] [Google Scholar]
- Saulsbury F. T., Winkelstein J. A., Yolken R. H. Chronic rotavirus infection in immunodeficiency. J Pediatr. 1980 Jul;97(1):61–65. doi: 10.1016/s0022-3476(80)80131-4. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Wells P. W. Rotavirus infection in lambs: studies on passive protection. Arch Virol. 1976;52(3):201–205. doi: 10.1007/BF01348017. [DOI] [PubMed] [Google Scholar]
- Sonza S., Holmes I. H. Coproantibody response to rotavirus infection. Med J Aust. 1980 Nov 1;2(9):496–499. doi: 10.5694/j.1326-5377.1980.tb100710.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T. N., Conner M. E., Graham D. Y., Estes M. K. Molecular characterization of three rabbit rotavirus strains. Arch Virol. 1988;98(3-4):253–265. doi: 10.1007/BF01322173. [DOI] [PubMed] [Google Scholar]
- Torres A., Ji-Huang L. Diarrheal response of gnotobiotic pigs after fetal infection and neonatal challenge with homologous and heterologous human rotavirus strains. J Virol. 1986 Dec;60(3):1107–1112. doi: 10.1128/jvi.60.3.1107-1112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Bernstein D. I., Shukla R., Young E. C., Sherwood J. R., McNeal M. M., Walker M. C., Schiff G. M. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis. 1989 Jan;159(1):79–88. doi: 10.1093/infdis/159.1.79. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Kelso N. E., Simpson T. F., Gaul S. K., Evans L. E., Babiuk L. Antigenic relationships among some bovine rotaviruses: serum neutralization and cross-protection in gnotobiotic calves. J Clin Microbiol. 1983 Aug;18(2):358–364. doi: 10.1128/jcm.18.2.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Zheng S. L., Rosen B. I., Knight N., Gourley N. E., Ramig R. F. Protection between different serotypes of bovine rotavirus in gnotobiotic calves: specificity of serum antibody and coproantibody responses. J Clin Microbiol. 1987 Jun;25(6):1052–1058. doi: 10.1128/jcm.25.6.1052-1058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., Kapikian A. Z., Mebus C. A. Induction of cross-reactive serum neutralizing antibody to human rotavirus in calves after in utero administration of bovine rotavirus. J Clin Microbiol. 1983 Sep;18(3):505–508. doi: 10.1128/jcm.18.3.505-508.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B. J., Han S. X., Yan Y. K., Liang X. R., Ma G. Z., Yang Y., Ng M. H. Development of neutralizing antibodies and group A common antibodies against natural infections with human rotavirus. J Clin Microbiol. 1988 Aug;26(8):1506–1512. doi: 10.1128/jcm.26.8.1506-1512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


